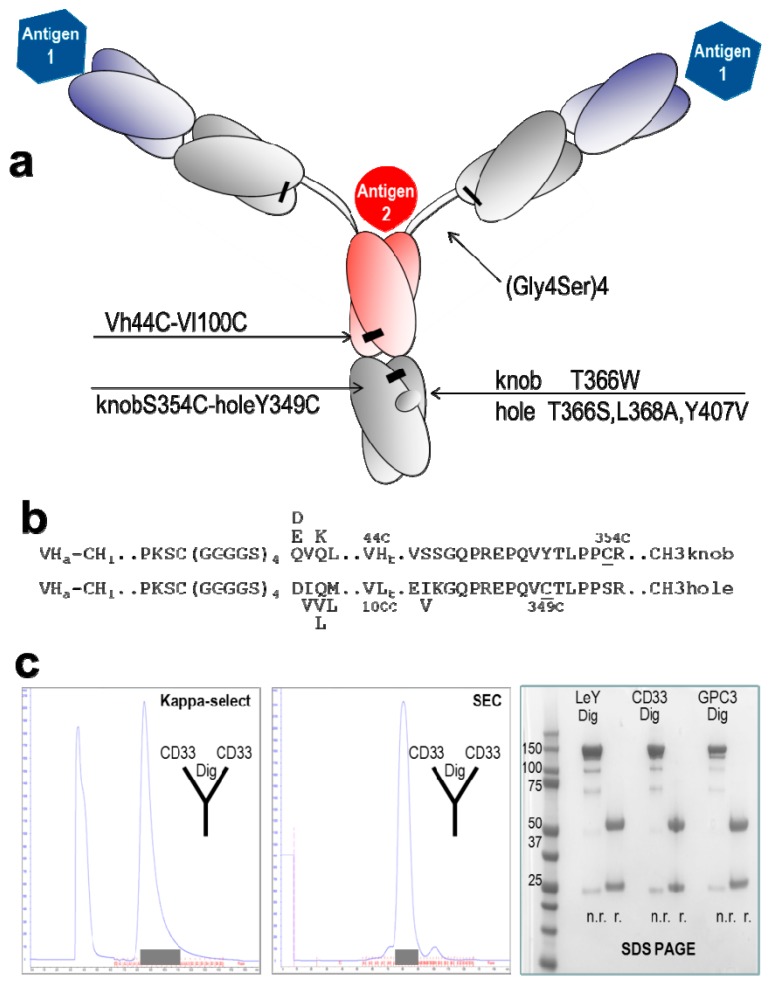
Figure 1.
Design and generation of TriFabs. (a) TriFabs have the IgG hinge replaced by linker peptides without disulfides, and the CH2 regions by VH or VL. Hetero-dimerization is achieved by disulphide-stabilized knob-into-hole CH3, and by introducing a H44-L100 disulphide in the Fv. Interchain disulfides that connect light and heavy chains and the engineered stem heterodimer are indicated by black bars; (b) Fusion sequences linking CH1 with VH or VL with CH3. The N-terminus of Dig-VH and GPC3-VH is QVQL, DVQL for LeY-VH, EVQL for CD33-VH. The N-terminus of Dig-VL is DIQM, GPC3-VL DVVM, LeY-VL DVLM and CD33-VL DIQL. The N-terminal elbow region of CH3 hole is EIKG for GPC3, LeY and Dig, and EVKG for CD33; (c) TriFabs are purified from cell culture supernatants by affinity chromatography with kappa-select (left panel, Protein A does not capture our TriFabs). After loading supernatants to the column (left peak in Figure 1c), TriFabs were eluted with 100 mM Glycine-buffer (pH 2.5), subsequently adjusted to pH 6.0–7.5 with 1 M Tris (pH 9.0). This is followed by size exclusion chromatography (middle panel). Shaded boxes indicate fractions containing properly folded TriFab. The composition and purity of TriFabs obtained by this simple two-step procedure is shown in the SDS PAGE without (n.r.) and with (r.) sample reduction (right panel). The purification profiles are exemplarily shown for TriFabs with CD33-CD33-Dig specificity. The purification and profiles of other TriFabs are described in the suppl. data section.