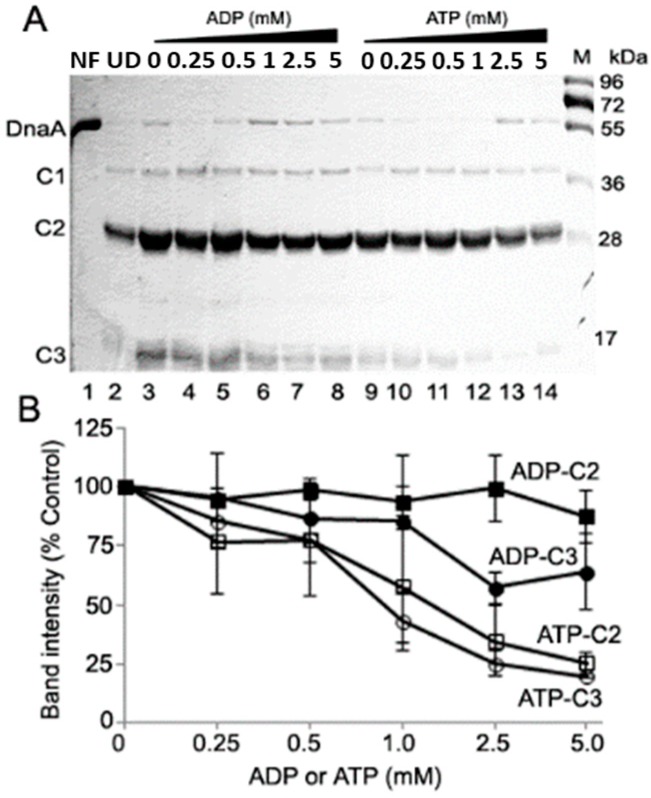
Figure 3.
ADP-DnaA and ATP-DnaA confers different resistance to chymotrypsin protease. (A) Nucleotide free, ADP and ATP-DnaA protein (1.5 μM) were subjected to proteolysis by chymotrypsin (protein: protease molar ratio of 4.0) for 30 min at 30 °C. Lane 1, Undigested (UD) nucleotide free DnaA; Lane 2, Nucleotide free DnaA (NF) treated with chymotrypsin; Lane 3, ADP-DnaA treated with chymotrypsin; Lanes 4–8, ADP-DnaA treated with chymotrypsin in the presence of 0.25, 0.5, 1.0, 2.5 and 5 mM ADP; Lane 9, ATP-DnaA treated with chymotrypsin; Lanes 10–14, ATP-DnaA treated with chymotrypsin in the presence of 0.25, 0.5, 1.0, 2.5 and 5 mM ATP; (B) A value of 100 corresponds to the intensity of the Fragment C2 and C3 present in the ADP-DnaA and ATP-DnaA (Lanes 3 and 9, i.e., no additional ADP or ATP in the reactions) respective to the band intensities present at each nucleotides concentration. Numbers denote the molecular weight in kDa. The values for band intensity corresponding to each proteolytic fragment were calculated by selecting a similar size area minus identical background from an empty part of the gel. Data shown here is the mean (±SD) from three independent experiments, including values from the representative SDS-PAGE.