Abstract
Interactions between biological vulnerability and environmental adversity are central to the pathophysiology of depression. Given evidence that the hypothalamic-pituitary-adrenal (HPA) axis influences biological responses to environmental events, in the current longitudinal study we examined HPA-axis functioning, negative life events, and their interaction as predictors of the first onset of depression. At baseline, girls ages 9 to 14 years provided saliva samples to assess levels of diurnal cortisol production, quantified by total cortisol production (area under the curve with respect to ground; AUCg) and the cortisol awakening response (CAR). We then followed these participants until they reached age 18 in order to assess their subsequent experience of negative life events and the onset of a depressive episode. We found that the influence of negative life events on the subsequent onset of depression depended on HPA-axis functioning at baseline. Specifically, negative life events predicted the onset of depression in girls with higher levels AUCg, but not in girls with lower levels of AUCg. In contrast, CAR did not predict the onset of depression either alone or in interaction with negative life events. These findings suggest that elevated total cortisol production in daily life potentiates susceptibility to environmental adversity and signals the need for early intervention.
Diathesis-stress models have been instrumental in furthering our understanding of depression (e.g., Flynn & Rudolph, 2007; Hammen, 2005; Monroe & Simons, 1991). Although researchers have reliably documented increased rates of the onset and recurrence of depression following major life events (e.g., Monroe & Hidjiyannakis, 2002; Monroe & Roberts, 1990), chronic stressors (e.g., Brown & Harris, 1986), and daily hassles (e.g., Lazarus & Folkman, 1984), many individuals do not develop depression following exposure to a stressful event (e.g., Bonanno, 2004). Thus, stressors themselves are not sufficient to induce a depressive episode. Instead, preexisting vulnerabilities significantly affect the ways in which individuals respond to acute stressors, thereby influencing their likelihood of developing a depressive episode (Hammen, 2005; Goodman & Gotlib, 1999).
Previous cross-sectional studies conducted by our research group and others have found that individuals at risk for depression show atypical cognitive and biological functioning (see reviews by Foland-Ross, Hardin, & Gotlib, 2013; Gotlib & Joormann, 2010). From this work, hypothalamic-pituitary-adrenal (HPA) axis activity has been identified as a key biological marker of risk for depression (Colich, Kircanski, Foland-Ross, and Gotlib, in press; Foland-Ross et al., 2013). For example, Colich et al. (in press) found that cortisol reactivity to stress predicted the onset of depression in a sample of adolescent girls without a history of psychopathology who were at a later stage of pubertal development. In the current study, we aimed to extend this work by examining diurnal cortisol production as a prospective predictor of depression onset.
Cortisol production has a strong diurnal pattern: cortisol levels rise rapidly after waking, reach a peak approximately 30 minutes later, and then fall throughout the day. Diurnal cortisol fluctuations are influenced by HPA-axis functioning (e.g., glucocorticoid receptor density, glucocorticoid sensitivity, and adrenal gland sensitivity), psychological variables, environmental stressors, and sleep-wake cycles (Fries, Dettenborn, & Kirschbaum, 2009; Sapolsky, 2000). Whereas moderate diurnal cortisol levels represent an adaptive response to environmental changes, excess cortisol production can stem from chronic HPA-axis activation, faulty negative feedback loops, or chronic stress exposure, and can affect key brain regions implicated in depression. The hippocampus, for example, has a high density of glucocorticoid receptors, and excess cortisol secretion has been shown to lead to neurotoxicity in this brain structure (Sapolsky, 2000). Excess cortisol production can also disrupt functioning in key emotion-relevant regions of the brain (e.g., the prefrontal cortex and amygdala) and thus interfere with the ability to cope effectively with future stressors (McEwen, 2008).
The cortisol awakening response (CAR) and cortisol area under the curve with respect to ground (AUCg) are two of the most commonly used indices of diurnal cortisol functioning. CAR measures the rapid increase in cortisol that occurs during the 30 minutes after awakening, and is particularly sensitive to the demands of the upcoming day. For example, CAR is steeper with greater perceived stress, on workdays than on weekends, and when there is higher stress early in the day (Fries, Dettenborn, & Kirschbaum, 2009). Importantly, CAR is distinct from total daily cortisol exposure, estimated as AUCg (Hill Golden et al., 2013). AUCg takes into account both the fluctuations in cortisol levels throughout the day (i.e., the change in cortisol levels from one time point to the next) and the overall magnitude of cortisol exposure (i.e., the distance of these measures from ground).
Researchers have documented that both CAR and AUCg are associated with elevated depressive symptoms in children and adults (Ehlert, Gaab, & Heinrichs, 2001; Guerry & Hastings, 2011; Holsboer, 2000). Although historically results have been equivocal (Knorr, Vinberg, Kessing, & Wetterslev, 2010), an increasing number of studies has found that, compared to never-depressed controls, currently depressed individuals exhibit a steeper CAR, higher peak cortisol in the morning, and higher overall cortisol levels across the day, as measured by AUCg or by single time-point estimates (Bhagwagar, Hafizi, & Cowen, 2005; Dienes, Hazel, & Hammen, 2013; Lopez-Duran, Kovacs, & George, 2009; Vreeburg et al., 2009). Atypical cortisol patterns have also been documented in individuals with a history of depression (e.g., Appelhof et al., 2006; LeMoult, Chen, Foland-Ross, Burley, & Gotlib, in press). These findings are consistent with the formulation that atypical HPA-axis function is a feature or ‘scar’ of the depressive episode. Importantly, however, cortisol patterns have also been posited to be a trait-like vulnerability factor that increases risk for the development of depressive episodes in youth; from this perspective, findings of atypical cortisol patterns in formerly depressed individuals may also represent long-standing difficulties in HPA-axis functioning.
Indeed, in several prospective investigations, atypical cortisol patterns have been found to predict depression in youth at risk for the disorder (Carnegie et al., 2014; Ellenbogen, Hodgins, Linnen, & Ostiguy, 2011; Goodyer, Bacon, Ban, Croudace, & Herbert, 2009; Halligan, Herbert, Goodyer, & Murray, 2007). For example, in a sample of 13- to 25-year-old male and female adolescent offspring of parents with or without a history of bipolar disorder, Ellenbogen and colleagues (2011) examined whether average cortisol levels across the day (i.e., at 60 minutes post-awakening, 3:00 pm, 8:00 pm, and bedtime) predicted the onset of an affective disorder up to six years later. Higher average cortisol levels across the day increased the likelihood that individuals would be diagnosed with an affective disorder over the follow-up period. Goodyer et al. (2009) also documented an association between cortisol levels and depression onset. Specifically, in at-risk male and female adolescents age 12 to 17 years at baseline, higher morning cortisol levels predicted the development of depression 12 months later. Similarly, Owens et al. (2014) examined a sample of boys ages 12 to 16 years at baseline and found that boys with elevated depressive symptoms and high levels of morning cortisol (collected at 8:00 am or 45 minutes after awakening) were at highest risk for future depression. Divergent results were reported, however, by Carnegie et al. (2014), who conducted a large longitudinal study of 15-year-old male and female adolescents, including adolescents with current depression and those who had a history of depression. Carnegie et al. found that neither CAR nor AUCg predicted the development of depression by age 18.
As a first step toward integrating diurnal cortisol and stressful life events in the prediction of depression, several researchers have assessed the independent effects of baseline cortisol and negative life events occurring between the baseline assessment and subsequent onset of depression. Goodyer, Tamplin, Herbert, and Altham (2000) were among the first to examine longitudinally the main effects of cortisol and negative life events on the development of depression. In a sample of male and female adolescents ages 12 to 16 years at baseline, Goodyer et al. examined whether baseline cortisol levels in the morning and evening and negative life events experienced between the baseline and 12 month follow-up assessment independently predicted the onset of depression at follow-up. Both cortisol levels at 8:00 am and negative life events predicted which adolescents developed depression; however, cortisol levels at 8:00 pm did not predict subsequent depression. Similar results were reported by Adam, Doane, Zinbarg, Mineka, Craske and Griffith (2009) in a sample of older male and female adolescents (ages 16 to 18 years at baseline) who were oversampled for high levels of neuroticism. Both steeper CAR at baseline and stressful life events experienced between baseline and follow-up independently predicted the development of a new clinically significant depressive episode one year later. Total cortisol production across the day (indexed via AUCg), however, did not contribute unique variance to the prediction of depression. Importantly, neither Goodyer et al. nor Adam et al. excluded adolescents with a history of psychopathology, complicating the interpretation of their findings. In addition, both studies examined only main effects of cortisol and negative life events, and did not examine the ‘diathesis-stress’ interaction of these two variables.
To date, only one study has examined whether the interaction of cortisol and stressful life events predicts the onset of depression, as a direct test of the diathesis-stress model. Vrshek-Schallhorn et al. (2013) collected cortisol levels at baseline from older male and female adolescents, ages 16 to 18 years, and assessed the occurrence of negative life events and a diagnosis of depression during up to four annual follow-up interviews. The authors examined whether CAR at baseline, stressful life events between baseline and follow-up, and their interaction predicted the onset of depression. Although both a steeper CAR and a higher number of stressful life events independently predicted the onset of depression, the interaction between CAR and stressful life events was not a significant predictor of depression. In contrast, AUCg did not predict depression as a main effect, and Vrshek-Schallhorn et al. did not examine the interaction between AUCg and stressful life events. Notably, adolescents were excluded from this study if they met criteria for current depression or posttraumatic stress disorder at baseline or had a history of dysthymic disorder, psychotic symptoms, or bipolar disorder. However, adolescents with any other current anxiety disorder and those with a lifetime history of depression were not excluded, which may have influenced the baseline CAR and its relation to future (recurrence of) depression. Extant data clearly demonstrate that HPA-axis functioning is influenced both by a history of depression (e.g., LeMoult et al., 2015) and by a diagnosis of a current or past anxiety disorder (e.g., Vreeburg et al., 2010; Mantella et al., 2010). Therefore, it is necessary to use diagnostically clean samples at baseline in order to ensure that HPA-axis functioning is not confounded by current or past psychopathology and to reveal whether cortisol and negative life events are antecedents, features, or scars of depression.
In sum, although several studies have examined the effects of cortisol production and negative life events on the onset of depression, only one investigation has explicitly tested the diathesis-stress model by examining the interaction between baseline cortisol and negative life events; however, results from this latter study are confounded by the inclusion of participants with a history of depression and current anxiety disorders. The primary goal of the current study was to address these limitations and build on this nascent literature by examining, for the first time, the interaction between baseline diurnal cortisol functioning and subsequent exposure to stressful life events as a predictor of the first onset of depression in a sample of young adolescents without a history of psychopathology. In the current study, we recruited a sample of young adolescent females, ranging in age from 9 to 14 years at baseline. Given gender differences in both patterns of salivary cortisol and rates of depression onset, we decided to reduce the heterogeneity of our sample by recruiting only female participants. We assessed participants’ salivary cortisol levels from waking through bedtime, allowing us to calculate both AUCg and CAR. We then followed these girls across the high-risk developmental period of adolescence through the age of 18, during which we regularly assessed the development of a clinically significant depressive disorder. Consistent with diathesis-stress models of depression, we predicted that both AUCg and CAR would interact with subsequent negative life events to predict the onset of a depressive disorder. Specifically, given evidence that the HPA axis plays a central role in regulating individuals’ stress response, we expected that elevated cortisol production at baseline would increase the degree to which stressful life events potentiated the onset of depression. Thus, we predicted that stressful life events would predict the onset of depression most strongly for those participants who exhibited greater AUCg or a steeper CAR.
Methods
Participants
Participants were 62 girls who were recruited as part of a larger longitudinal study of girls at risk for depression. At the time of entry into the study, participants were between 9 and 14 years of age and had no current or past Axis I disorder. In order to ensure that a sufficient number of girls would develop depression over the follow-up period, we recruited both daughters of mothers who had no history of depression (n=32) and daughters of mothers who had a history of recurrent depression during their daughters’ lifetime (n=30). Participants were recruited from the local community through internet and print advertisements or through the Department of Psychiatry and Behavioral Sciences at Stanford University. The study was approved by the Stanford University Institutional Review Board; participants and their parents gave assent and informed consent, respectively. Participants were screened for initial inclusion/exclusion through a telephone interview and were then invited to the laboratory for in-person interviews and assessments. No participants had any reported major medical complications, health conditions known to interfere with HPA-axis activity (including pregnancy and endocrine disorders, per Kudielka, Hellhammer, & Wust, 2009), head trauma, past or present Axis I disorder, history of taking a psychotropic medication, or learning disorder, and all were fluent in English. Of the original 190 girls who were part of the larger study, 81 girls were eligible for inclusion in the current study based on the inclusion and exclusion criteria described above and on having provided samples of diurnal cortisol. Of this sample, 19 dropped out of the study; we report here on the remaining 62 girls who developed a subsequent episode of MDD or who we have followed through the age of 18 years to classify as having not developed an episode of MDD (i.e., non-converted). Participants who were not included in the final sample were slightly younger (M=11.64, SD=1.56) than those who were included in the final sample (M=12.31, SD=1.36), t(119)=2.48, p=.014. Otherwise, however, clinical and demographic characteristics of the 62 participants included in the current study do not differ from those of the rest of the sample, ps>.05.
To date, one longitudinal study has been conducted with this sample to examine the onset of depression. Colich et al. (in press) examined whether pubertal status moderates the relation between cortisol reactivity to a laboratory stressor and the onset of depression in a sample of 89 girls. It is important to note that data from the current study represent a test of an independent, a priori hypothesis, examining the influence of diurnal cortisol production on risk for depression in the smaller subset of participants who provided samples of diurnal cortisol.
Time 1 Assessments
Interviews
At Time 1 (baseline), participants came to Stanford University to complete a structured clinical interview to assess current and lifetime psychopathology. Trained interviewers assessed participants’ diagnostic status by administering the Schedule for Affective Disorders and Schizophrenia (K-SADS-PL; Kaufman, Birmaher, Brent, & Rao, 1997) to both the girls and their mothers (about their daughters). A different interviewer administered the Structured Clinical Interview for DSM (SCID; First, Spitzer, Gibbon, & Williams, 2000) to the mothers in order to assess the presence of current and past psychopathology. To assess inter-rater reliability for the participant interviews in this study, an independent rater who was blind to group membership evaluated a portion of SCID and K-SADS-PL interviews. In all cases, diagnoses and group assignment matched those given by the original interviewer, K =1.00. In order to be eligible for study inclusion, girls could not have met clinical or subclinical levels of any Axis I disorder at any time in their life based on either mother or daughter report. Other inclusion and exclusion criteria were also confirmed at this time.
Self-report measures
Participants completed the Children’s Depression Inventory – Short Form (CDI-S; Kovacs, 1992), Anxiety Sensitivity Index for Children (ASIC; Laurent et al., 1998), Multidimensional Anxiety Scale for Children (MASC; March, Parker, Sullivan, Stallings, & Conners, 1997), and the Tanner Staging questionnaire (Marshall & Tanner, 1968). The CDI-S is a 10-item self-report measure of depressive symptomatology for children between 8 and 17 years of age, and has been used widely in studies of clinical and non-clinical children and adolescents. Higher CDI-S scores reflect greater depressive symptoms. The ASIC is a 16-item self-report measure of anxiety sensitivity in children, and can be used with both clinical and non-clinical populations. A composite score for the ASIC was calculated by averaging across the 16 items; higher scores reflect greater anxiety sensitivity. The MASC is a 39-item self-report measure used to assess symptoms of anxiety in children. The total score is calculated by summing across the 39 items and indicates the overall extent to which the child is experiencing symptoms of anxiety. Participants also completed the Tanner Staging questionnaire to assess self-reported pubertal status, which has been found to correlate highly with physicians’ physical examinations of pubertal development (e.g., Shirtcliff et al., 2009). Participants were asked to indicate their developmental stage using schematic drawings of two secondary sex characteristics (breast and pubic hair). A composite score of these two ratings was used to index girls’ pubertal stage at baseline. The CDI-S was included to confirm that neither group had clinically meaningful depressive symptoms at baseline and to control for individual differences in baseline depressive symptoms when predicting first onset of depression. The ASIC, MASC, and Tanner Staging questionnaires were included to use as possible covariates in the event that girls who developed depression differed from those who did not develop depression in scores on these baseline measures. One girl did not complete the ASIC, two girls did not complete the MASC, and four girls did not complete the Tanner Staging questionnaire.
Diurnal cortisol collection
Within two weeks of this baseline session, participants completed two days of at-home diurnal cortisol collection. Participants were instructed not to eat, drink, or brush their teeth for two hours before each sample. Participants used Salivette kits (Sarstedt, Germany) to collect a saliva sample at each of the following four time points: immediately upon waking, 30 minutes post-waking, mid-afternoon (approximately 3:00 pm), and 30 minutes before bedtime. Participants recorded the actual time of cortisol collection on a separate piece of paper and these data were used in all calculations. For a random subset of participants (n=26), smart caps were used to track the times at which they opened the bottle to retrieve the Salivette. Cortisol collection times obtained from smart caps did not differ significantly from the self-reported collection times, ts<1.85, ps>.05. After saliva collection, participants stored Salivettes in their home freezer until they could be transferred to the −20 °F freezer at Stanford University. Cortisol levels were assayed by luminescence immunoassay reagents using a commercial kit from Immuno-Biological Laboratories Inc. (Hamburg, Germany), and the assay sensitivity was set at 0.015 μg/dl. Samples were assayed together in large batches to control for interassay error, and control samples were included to evaluate variability. Because the salivary cortisol data remained positively skewed after logarithmic and other transformations, we winsorized values 2 standard deviations (SDs) above the mean to the 2-SD value. We then calculated girls’ CAR (slope of waking to 30 minutes post-waking) and total cortisol production throughout the day (AUCg) using methods recommended by Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (2003). To obtain the most precise cortisol estimates for each participant, we used the actual rather than the expected time of cortisol collection in these calculations.
Follow-up Assessments
Interviews
To monitor the course of depressive symptoms and the possible onset of depression through the age of 18, girls returned to the laboratory approximately every 18 months. Interviewers administered the K-SADS interview to participants under the age of 18 and the SCID to participants who were age 18. Diagnostic status for clinical depression required meeting DSM-IV diagnostic criteria for a unipolar depressive disorder with no history of manic, hypomanic, or mixed episodes. Of the original 62 participants, 33 met criteria for a depressive disorder before the age of 18, 26 of whom met DSM-IV criteria for Major Depressive Disorder and 7 of whom met criteria for Depressive Disorder, Not Otherwise Specified (defined as the endorsement of three or four symptoms of depression, present for at least two weeks, with clinically significant impairment or distress). Importantly, all depressive episodes included in the model represent participants’ first onset of depression; data collected in sessions after a participant experienced a depressive episode were not included. In contrast, girls who did not develop depression were followed until they reached age 18. Thus, as expected, girls who experienced a depressive episode completed fewer follow-up visits (M=2.15, SD=0.94) than did girls who did not experience a depressive episode (M=3.07, SD=1.03), t(60)=3.66, p=.001.
Self-report measures
At each follow-up assessment, participants reported on the life events that they had experienced since the previous assessment using the Life Events Checklist (Johnson & McCutcheon, 1980), which has been designed for children and adolescents, or using the parallel Life Events Survey if the participant was age 18 (Sarason, Johnson, & Siegel, 1978). Both measures have been shown to have adequate psychometric properties in comparable samples (Brand & Johnson, 1982; Sarason et al., 1978). Participants reported on the presence of a total of 50 dependent and independent life events (e.g., death of family member, parents separated, breaking up with boyfriend/girlfriend, trouble with teacher, trouble with classmates) and rated the subjective impact of negative life events on a four-point scale. Based on recommend scoring procedures (Brand & Johnson, 1982; Sarason et al., 1978), we summed the absolute value of participants’ subjective impact ratings for all negative life events that were endorsed in order to obtain a total negative life events score. Negative life events scores were divided by the number of months included in the assessment interval in order to adjust for differences among participants in the exact amount of time since the last assessment. Therefore, negative life events scores reflect the average subjective impact of negative life events per month since the previous assessment. Negative life events scores were examined for the period immediately prior to the onset of depression for those who developed depression, or for the matched follow-up assessment for those participants who did not develop depression. Specifically, for those individuals who developed depression, we examined negative life event scores for the period immediately prior to depression onset (e.g., for a girls who developed depression at third follow-up assessment, negative life event scores between the second and third follow-up assessment). For those individuals who did not develop depression, we calculated negative life events for the matched follow-up session (i.e., in this example, from the second to the third follow-up assessment).
Planned Analyses
The primary analyses involved a series of hierarchical logistic regressions, with the baseline cortisol measure of interest (AUCg or CAR) and negative life events (Step 1), and the interaction of cortisol and negative life events (Step 2) regressed on a dummy variable that reflected the presence or absence of experiencing a depressive disorder by age 18. Several covariates were included in Step 1 of the regression models: mothers’ history of depression at baseline, children’s age at baseline, CDI-S scores at baseline, and time between baseline and follow-up. Given concerns of multicollinearity, separate logistic regression analyses were conducted for AUCg and CAR. Continuous variables were grand mean centered and dichotomous variables were effect coded. Analyses were conducted using HLM software version 6.01 (Randenbush, Bryk, & Congdon, 2004) using restricted maximum likelihood to provide less biased estimates of variance (Randenbush & Byrk, 2002).
Results
Participant Characteristics
Demographic and clinical characteristics of the participants are presented in Table 1. “Converted” girls (i.e., those who developed depression by age 18) did not differ significantly in their pubertal stage at baseline from “non-converted” girls, t(56)=0.47, p=.640, d=0.124. Converted and non-converted girls also did not differ significantly in their baseline CDI-S scores, t(60)=1.71, p=.093, d=0.432, levels of anxiety sensitivity, t(59)=1.07, p=.289, d=0.275, scores on the MASC, t(58)=0.65, p=.521, d=0.168, or time between baseline and follow-up ratings of life events, t(60)=0.12, p=.904, d=0.031. There was also no significant group difference in ethnic composition, χ2(4, N=62)=7.03, p=.134, V=0.337. Compared to non-converted girls, converted girls were slightly younger at baseline, t(60)=2.78, p=.007, d=0.707, and, not surprisingly, were more likely to have a mother with a history of depression, χ2(1, N=62)=12.83, p<.001, V=.455. Therefore, we included girls’ age at baseline and maternal history of depression, among other variables, as covariates.1
Table 1.
Participant Characteristics
| Variable | Converted (N=33) | Non-Converted (N=29) | p-value |
|---|---|---|---|
| Baseline age, M(SD) | 11.88(1.39) | 12.79(1.18) | 0.007 |
| Baseline Tanner Stage, M(SD) | 3.17(0.94) | 3.29(0.94) | 0.640 |
| Caucasian, % | 70% | 72% | 0.134 |
| Maternal history of depression, % | 70% | 24% | <.001 |
| Baseline CDI-S, M(SD) | 2.36(2.79) | 1.38(1.45) | 0.093 |
| Baseline ASIC, M(SD) | 10.15(5.42) | 8.68(5.28) | 0.289 |
| Baseline MASC, M(SD) | 40.42(14.78) | 38.19(11.36) | 0.521 |
| Baseline salivary cortisol level, M(SD) | |||
| Wake | 0.56(0.32) | 0.46(0.21) | 0.145 |
| Wake + 30 | 0.75(0.34) | 0.74(0.27) | 0.882 |
| Afternoon | 0.21(0.15) | 0.18(0.11) | 0.427 |
| Bedtime | 0.07(0.06) | 0.05(0.05) | 0.365 |
| Time baseline to follow-up (months), M(SD) | 49.20(20.58) | 49.82(19.67) | 0.904 |
| Negative life events scores, M(SD) | 0.54(0.41) | 0.36(0.38) | 0.075 |
Note. CDI-S=Children’s Depression Inventory-Short Form; ASIC=Anxiety Sensitivity Index for Children
Pattern of Diurnal Cortisol Production
Diurnal cortisol data are presented in Table 1. Overall, the expected pattern of diurnal cortisol production was observed. Cortisol levels increased within the first 30 minutes of awakening, tpaired(61)=5.73, p<.001, d=0.731, and then declined significantly throughout the day, tspaired(61)>8.64, ps<.001, ds≤3.000. Negative life event scores were not correlated significantly with either AUCg, r(62)=−0.116, p=.370, r2=0.013, or CAR, r(62)=−0.106, p=.412, r2=0.011.
Cortisol AUCg by Negative Life Events in Predicting First Onset of Depression
Table 2 presents the results of the logistic regression analyses in which the first onset of depression was predicted by AUCg, negative life events, mothers’ history of depression, girls’ age at baseline, CDI-S score at baseline, and time between baseline and follow-up (in Step 1), and the interaction of AUCg and negative life events (in Step 2). Girls whose mothers had a history of depression were more likely to develop depression by age 18, β=1.07, p=.002, OR=2.91. In addition, girls’ age at baseline was negatively associated with developing depression, β=−1.61, p < .001, OR=0.20. In contrast, neither baseline CDI-S scores, β=0.68, p=.142, OR=1.98, nor time between baseline and follow-up, β=−0.42, p=.238, OR=0.66, uniquely predicted the development of depression. After accounting for these covariates, neither AUCg, β=0.62, p=.053, OR=1.85, nor negative life events, β=0.32, p=.458, OR=1.38, were found to predict the development of depression. When the interaction between AUCg and negative life events was added in Step 2, however, it significantly predicted the development of depression, β=0.93, p=.030.2
Table 2.
Predicting Depression Onset
| AUCg
|
CAR
|
|||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | t | p | β | SE | t | p | |
| Step 1 | ||||||||
| Intercept | 0.22 | 0.35 | 0.71 | 0.482 | 0.27 | 0.33 | 0.80 | 0.427 |
| Maternal history of depression | 1.07 | 0.33 | 3.27 | 0.002 | 1.21 | 0.37 | 3.27 | 0.002 |
| Baseline age | −1.61 | 0.39 | 4.17 | < .001 | −1.47 | 0.47 | 3.14 | 0.003 |
| Baseline CDI-S | 0.68 | 0.46 | 1.49 | 0.142 | 0.87 | 0.54 | 1.62 | 0.111 |
| Time baseline to follow-up | −0.42 | 0.35 | 1.19 | 0.238 | −0.13 | 0.28 | 0.45 | 0.654 |
| Cortisol | 0.62 | 0.31 | 1.97 | 0.053 | −0.37 | 0.34 | 1.09 | 0.281 |
| Negative life events | 0.32 | 0.43 | 0.75 | 0.458 | 0.17 | 0.49 | 0.35 | 0.725 |
| Step 2 | ||||||||
| Cortisol x Negative life events | 0.93 | 0.42 | 2.23 | 0.030 | 0.05 | 0.22 | 0.21 | 0.833 |
Note: CDI-S=Children’s Depression Inventory-Short Form; AUCg=Area under the curve with respect to ground; CAR=Cortisol awakening response
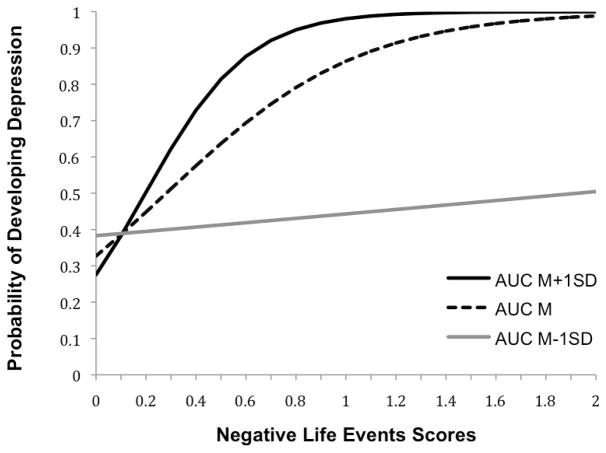
Figure 1 depicts the nature of the interaction between AUCg and negative life events in predicting conversion. For participants with lower AUCg production (1 SD below the mean; M−1SD=157.99), negative life event scores were not related to the probability of conversion, OR=1.10. In contrast, for participants with higher AUCg production (1 SD above the mean; M+1SD=436.98), higher negative life event scores were strongly predictive of the development of depression, OR=7.02. In fact, as presented in Figure 1, at the mean level of negative life event scores (M=0.45), girls with AUCg production 1 SD below the mean had a 41% chance of developing depression, whereas girls with AUCg production 1 SD above the mean had a 78% chance of developing depression. Thus, girls who produced more cortisol throughout the day were more susceptible to developing depression after experiencing negative life events than were girls who produced less cortisol throughout the day.
Figure 1.
Likelihood of developing depression as a function of diurnal cortisol area under the curve to ground (AUCg) and negative life event scores.
CAR by Negative Life Events in Predicting First Onset of Depression
Table 2 presents the results of the logistic regression analyses in which conversion was predicted by CAR, negative life events, and their interaction. The covariates included in this model were the same as those included in the model with AUCg (maternal history of depression, β=1.21, p=.002, OR=3.35, age at baseline, β=−1.47, p=.003, OR=0.23, baseline CDI-S score, β=0.87, p=.111, OR=2.39, time between baseline and follow-u, β=−0.13, p=.654, OR=0.88). After accounting for these covariates, neither CAR, β=−0.37, p=.281, OR=0.69, nor negative life events, β=0.17, p=.725, OR=1.19, predicted conversion. When the interaction between CAR and negative life events was entered in Step 2, it was not a significant predictor of the development of depression, β=0.05, p=.833.
Discussion
In this direct test of the diathesis-stress model of depression, we examined the interaction of diurnal HPA-axis functioning at baseline and subsequent experiences of negative life events in predicting the first onset of depression in a sample of female adolescents who had no history of psychopathology. We found that the influence of negative life events on the subsequent onset of depression depended on baseline AUCg production, but not on CAR. Specifically, girls with greater AUCg at baseline were more susceptible to developing depression after experiencing negative life events than were girls with lower AUCg. These findings suggest that only in the context of greater total diurnal cortisol production does negative life events potentiate risk for depression. Recruiting a sample of participants who were diagnostically clean at baseline and assessing them over an extended time frame allowed us to test whether the interaction between cortisol production and negative life events reflected a risk factor for the onset of depression rather than a scar or feature of the disorder. The clinical importance of determining markers of risk for depression is underscored by promising results from early interventions for this disorder (Gladstone, Beardslee, & O’Connor, 2011), compared with the difficulty of treating adolescent depression after its onset (Kessler et al., 2003; March et al., 2004). Indeed, early intervention may ultimately reduce the incidence of depression and lessen the substantial personal and societal costs of this debilitating disorder (Murray & Lopez, 1996; Stewart, Ricci, Chee, Hahn, & Morganstein, 2003).
Our findings help to reconcile a literature that has historically been mixed. Whereas some studies have found that total cortisol across the day predicts the onset of depression as a main effect (Ellenbogen et al., 2011), others have not (Adam et al., 2009; Vrshek-Schallhorn et al., 2013). Similarly, whereas CAR was found to predict the onset of depression in one sample (Adam et al., 2009; Vrshek-Schallhorn et al., 2013), it did not in another sample (Carnegie et al., 2014). These equivocal results may be due to the fact that all previous studies included participants who had current and/or past psychopathology. Given that individuals with current anxiety disorders or past depression exhibit abnormalities in diurnal cortisol production (Appelhof et al., 2006; LeMoult et al., in press), even a history of psychopathology may alter baseline HPA-axis functioning in otherwise asymptomatic individuals, thereby complicating interpretation of these prior findings. For example, when individuals with psychopathology are included in the sample, baseline cortisol abnormalities may serve as a marker of the presence of other forms of psychopathology; findings, therefore, might be due to anxiety or past depression – rather than to specific cortisol patterns per se – increasing risk for the onset of depression (Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993; Stein, Fuetsch, Müller, Höfler, Lieb, & Wittchen, 2001). Moreover, confounding the simultaneous testing of predictors of first onsets and recurrences of depression may obscure findings, particularly given that researchers have demonstrated that different variables predict the first depressive episode compared to subsequent episodes (Lewinsohn, Allen, Seeley, & Gotlib, 1999). In addition, the current study included female adolescents exclusively, whereas the majority of past research has included both males and females (e.g., Adam et al., 2009; Vrshek-Schallhorn et al., 2013). Including only female adolescents reduced the heterogeneity of our sample and, given gender differences in HPA functioning, could have eliminated a confounding factor.
This is the first study to test whether the interaction of AUCg and negative life events predicts the onset of depression. Thus, with respect to AUCg, mixed results of previous studies may be due to unexamined interactions between total cortisol production and experiences of negative life events, as findings from the current study suggest. This interpretation is consistent with diathesis-stress models of depression that posit that preexisting vulnerability factors, such as atypical HPA-axis function, interact with environmental stressors to potentiate risk for depression (e.g., Flynn & Rudolph, 2007; Hammen, 2005; Monroe & Simons, 1991). For example, Goodman and Gotlib (1999) posit that chronic activation of the HPA axis impairs individuals’ ability to cope effectively with stress. Although the precise mechanisms through which greater AUCg potentiates susceptibility to negative life events are not yet clear, Goodman and Gotlib noted that children who demonstrate chronic activation of the HPA-axis are hyper-responsive to environmental challenges. Moreover, because AUCg is measured with respect to ground, this metric assesses the total amount of cortisol to which individuals are exposed throughout the day. Higher total levels of cortisol can disrupt functioning in key emotion-relevant regions of the brain and interfere with the ability to cope with future stressors (Hinkelmann et al., 2009; Schuhmacher et al., 2012). Thus, greater AUCg at the baseline assessment might lead to biological disruptions that exacerbate individuals’ responses to subsequent negative life events and hinder their ability to use appropriate emotion regulation strategies to cope with these stressors. The equivocal literature on cortisol and depression may also be due to other unexamined moderators, such as pubertal status, gender, or history of psychopathology. Colich et al. (in press), for example, found that the onset of depression was predicted by cortisol hyporeactivity to stress in girls who were earlier in pubertal development (Tanner stage ≤ 2), but by cortisol hyperreactivity to stress in girls who were later in pubertal development (Tanner stage ≥ 3.5). Although the current study would have been underpowered in testing whether the interaction of diurnal cortisol production, negative life events, and puberty predicted the onset of depression, future research could profitably examine the nature of interactions among these variables.
Although we predicted that both AUCg and CAR would moderate risk for depression in the context of negative life events, it is informative that our interaction finding was specific to AUCg. Several investigators have found that AUCg and CAR reflect different biological processes (Golden et al., 2013; Schmidt-Reinwald et al., 1999). Specifically, whereas AUCg indexes total cortisol exposure throughout the day and is sensitive to multiple environmental demands, Wihelm, Born, Kudielka, Schlotz, and Wust (2007) posited that CAR reflects sensitivity of the adrenal cortex to awakening, which begins even prior to awakening and is affected by exposure to light and activity of the suprachiasmatic nucleus. Thus, it is possible that AUCg is a more appropriate index of sensitivity to environmental stressors than is CAR. In addition, compared to CAR, AUCg may be influenced more strongly by efficient functioning of HPA-axis negative feedback loops (Kudielka, Bellingrath, & Hellhammer, 2006; Schmidt-Reinwald et al., 1999), which are critical for down-regulating HPA-axis activity when elevated cortisol levels have been detected. Therefore, greater AUCg at baseline might be an early marker of inefficient functioning of these negative feedback loops, which might exaggerate individuals’ reactivity to future stressors.
It is noteworthy that rates of conversion in this sample were higher than in the general population (Kessler et al., 2005). This reflects several strategic decisions that we made in order to optimize our ability to examine predictors of depression over the follow-up period. First, the current sample was composed exclusively of girls, who have substantially higher rates of depression than do boys (Kessler et al., 2005). Second, based on findings of higher rates of depression in offspring of parents with a history of the disorder (Gotlib & Colich, 2014), we included in this study a subsample of girls whose mothers had experienced recurrent episodes of depression. Third, we defined conversion as meeting criteria for Major Depressive Disorder or Depressive Disorder Not Otherwise Specified in order to capture all diagnosable and clinically significant depressive disorders. This decision is consistent with clinical practice and the increasing importance placed on assessing a range of related clinical syndromes, such as the perspective exemplified by the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC). Finally, we recruited a younger sample of adolescents than had been used in other studies, and we followed these participants across the critical developmental window of adolescence, a period when individuals are particularly susceptible to developing depression (Anderson & Teicher, 2008).
We should note four limitations of the current study. First, we used a self-reported, rather than interview-based, measure of negative life events. Although interview-based measures of life stress provide less biased estimates of the amount or intensity of life stress, self-report measures of stress have been shown to be important for understanding both the onset and treatment of depression (Yip, 2004). Nevertheless, in the future, researchers should examine whether similar findings are obtained when an interview-based measure of negative life events is administered. Second, the measure of stressful life events used in this study did not allow us to differentiate the effects of dependent from independent negative life events (Hammen, 2006). Future studies should examine whether the relations reported here are obtained for both types of events. Third, although a subset of participants used smart caps to electronically track the time at which they opened the bottle to retrieve the Salivette, we did not obtain an independent measure of participants’ wake time in order to confirm that the first sample was taken immediately upon awakening. This is particularly relevant to findings related to CAR, which is highly sensitive to accurate sampling times. Finally, it is possible that the current study was underpowered to detect a significant interaction between cortisol and negative life events. This null finding should, therefore, be interpreted with caution and replication of the current findings within a larger sample is warranted. That the interaction of AUCg and negative life events was significant despite this potential low level of power, however, attests to the strength of this finding.
Despite these limitations, the current study is important in directly testing the diathesis-stress model by investigating, for the first time, the interactions of baseline HPA-axis functioning and stressful life events in predicting onset of depression in female adolescents who, at baseline, had no history of psychopathology. Importantly, the current study included girls at high and low risk for depression, and we found no evidence that these two groups of girls differed significantly with respect to the relation among cortisol, negative life events, and depression onset. Thus, although it is important that these findings be replicated in unselected samples, it is reasonable to posit that these findings generalize across high- and low-risk samples. The results of this study contribute to our understanding of biological risk for depression and demonstrate that individuals who exhibit atypical cortisol production across the day (measured by AUCg) are particularly susceptible to developing depression following negative life events. This finding has important implications for prevention efforts, suggesting that girls who demonstrate greater total diurnal cortisol production will benefit most from early intervention efforts. In this context, interventions using cognitive-behavioral and/or interpersonal approaches to decrease subjective reactivity to stressors and to improve emotion regulation and coping skills are likely to be particularly helpful (Gladstone et al., 2011). It is imperative that we continue to elucidate biomarkers of risk for depression so that we can identify which individuals are at greatest need for targeted interventions and reduce the significant personal and societal costs of this debilitating disorder.
LAY SUMMARY.
The current study examined whether individual differences in production of the primary stress hormone, cortisol, affects the degree to which negative life events predict the first onset of depression in adolescent girls. Findings suggest that girls who produced more cortisol throughout the day were more susceptible to developing depression after experiencing negative life events than were girls who produced less cortisol throughout the day.
Acknowledgments
We thank Maria Lemus and Kirsten Gilbert for their assistance in scheduling and running the participants. This research was supported by National Institute of Mental Health (NIMH) Grants F32-MH102013 (to Dr. Joelle LeMoult), T32-MH019938 (to Dr. Sarah Ordaz), F32-MH096385 (to Dr. Katharina Kircanski), K23-MH085919 (to Dr. Manpreet Singh), and R01-MH59259 (to Dr. Ian Gotlib), the Brain & Behavior Research Foundation (formerly NARSAD; Young Investigator Award 22337 to Dr. Joelle LeMoult, Young Investigator Award 20814 to Dr. Katharina Kircanski, and Distinguished Investigator Award to Dr. Ian Gotlib), and the Klingenstein Third Generation Foundation (Fellowship in Depression to Dr. Sarah Ordaz).
Footnotes
Girls whose mothers had a history of depression did not differ significantly from girls whose mothers did not have a history of depression on any of the demographic or clinical characteristics assessed, including pubertal stage, age at baseline, ethnicity, baseline CDI-S scores, baseline anxiety sensitivity, time between baseline and follow-up life event ratings, negative life events scores, or cortisol production at baseline, ps > .05.
We tested whether the relations among cortisol (AUCg or CAR), negative life events, and conversion differed as a function of maternal history of depression by including in the logistic regression analyses interactions among the cortisol measure of interest (AUCg or CAR), negative life events, and maternal history of depression. The interaction of cortisol, negative life events, and maternal history of depression was not significant in either analysis, ps>.05. We also conducted the hierarchical logistic regression analyses including ASIC scores as a covariate in Step 1. Baseline ASIC scores did not predict the development of depression in either the AUCg or CAR analyses, ps>.050, and the interaction between AUCg and negative life events remained significant when it was entered in Step 2, p=.023. We included MASC scores as a covariate in Step 1 of the hierarchical logistic regressions. Baseline MASC scores did not predict the development of depression in the logistic regression focused on AUCg or CAR, ps>.050, and the interaction between AUCg and negative life events remained significant when it was entered in Step 2, p=.012. We also we calculated the slopes from 30-minutes post-waking to mid-afternoon (slope from cortisol sample 2 to 3), from 30-minutes post-waking to 30 minutes before bedtime (slope from cortisol sample 2 to 4), and from mid-afternoon to 30 minutes before bedtime (slope from cortisol sample 3 to 4). Separate regression analyses were conducted for each slope calculation. None of the main effects of slope nor interactions between slope and negative life events were significant, ps>.05.
References
- Abramson LY, Alloy LB, Hogan ME, Whitehouse WG, Donovan P, Rose DT, et al. Cognitive vulnerability to depression: Theory and evidence. In: Leahy RL, Dowd ET, editors. Clinical Advances in Cognitive Psychotherapy: Theory and Application. New York: Springer Publishing Co; 2002. pp. 75–92. [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, Schene AH. Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression) Biological Psychiatry. 2006;59(8):696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Brand AH, Johnson JH. Note on reliability of the Life Events Checklist. Psychological Reports. 1982;50(3c):1274–1274. [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182(1):54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Bonnanno GA. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Stressor, vulnerability and depression: A question of replication. Psychological Medicine. 1986;16(4):739–744. doi: 10.1017/s0033291700011740. [DOI] [PubMed] [Google Scholar]
- Carnegie R, Araya R, Ben-Shlomo Y, Glover V, O’Connor TG, O’Donnell KJ, Lewis G. Cortisol awakening response and subsequent depression: Prospective longitudinal study. The British Journal of Psychiatry. 2014;204(2):137–143. doi: 10.1192/bjp.bp.113.126250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47(3):864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dienes KA, Hazel NA, Hammen CL. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology. 2013;38(6):927–940. doi: 10.1016/j.psyneuen.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Levine S. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11(2):189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus–pituitary–adrenal axis. Biological Psychology. 2001;57(1):141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Linnen AM, Ostiguy CS. Elevated daytime cortisol levels: a biomarker of subsequent major affective disorder? Journal of Affective Disorders. 2011;132(1):265–269. doi: 10.1016/j.jad.2011.01.007. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. New York: Biometrics Research, New York State Psychiatric Institute; Nov, 2002. SCID-I/P W/PSY SCREEN. [Google Scholar]
- Flynn M, Rudolph K. Perceptual asymmetry and youths’ responses to stress: Understanding vulnerability to depression. Cognition & Emotion. 2007;21:773–788. doi: 10.1080/02699930600824635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Hardin MG, Gotlib IH. Neurobiological markers of familial risk for depression. In: Cowen PJ, Sharp T, Lau JYF, editors. Behavioral Neurobiology of Depression and Its Treatment: Current Topics in Behavioral Neurosciences. Berlin: Springer-Heidelberg; 2013. pp. 181–206. [DOI] [PubMed] [Google Scholar]
- Gladstone TR, Beardslee WR, O’Connor EE. The prevention of adolescent depression. Psychiatric Clinics of North America. 2011;34(1):35–52. doi: 10.1016/j.psc.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SH, Sánchez BN, Wu M, Champaneri S, Diez Roux AV, Seeman T, Wand GS. Relationship between the cortisol awakening response and other features of the diurnal cortisol rhythm: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2013;38(11):2720–2728. doi: 10.1016/j.psyneuen.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Bacon A, Ban M, Croudace T, Herbert J. Serotonin transporter genotype, morning cortisol and subsequent depression in adolescents. The British Journal of Psychiatry. 2009;195(1):39–45. doi: 10.1192/bjp.bp.108.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Tamplin A, Herbert J, Altham PME. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. The British Journal of Psychiatry. 2000;177(6):499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. 3. New York: Guilford Press; 2014. pp. 240–258. [Google Scholar]
- Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Family Psychology Review. 2011;14(2):135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62(1):40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress generation in depression: Reflections on origins, research, and future directions. Journal of Clinical Psychology. 2006;62(9):1065–1082. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Brown GW, Cleary SE, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. The British Journal of Psychiatry. 2000;177(6):505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Johnson JH, McCutcheon SM. Assessing life stress in older children and adolescents: Preliminary findings with the Life Events Checklist. Stress and anxiety. 1980;7:111–125. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for affective disorders and Schizophrenia for school-age children—present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29(2):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory: Manual. Multi-Health Systems; 1992. [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35(9):1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Bellingrath S, Hellhammer DH. Cortisol in burnout and vital exhaustion: An overview. Giornale Italiano di Medicina Lavoro Ergonomia. 2006;28:34–42. [PubMed] [Google Scholar]
- Laurent J, Schmidt NB, Catanzaro SJ, Joiner TE, Jr, Kelley AM. Factor structure of a measure of anxiety sensitivity in children. Journal of anxiety Disorders. 1998;12(4):307–331. doi: 10.1016/s0887-6185(98)00017-6. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: differential processes of psychosocial risk. Journal of Abnormal Psychology. 1999;108(3):483. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34(9):1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Lenze EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33(6):773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Severe J. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA. 2004;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual Review of Medicine. 1968;19(1):283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583(2):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Hadjiyannakis K. The social environment and depression: Focusing on severe life stress. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. New York: Guilford; 2002. pp. 314–340. [Google Scholar]
- Monroe SM, Roberts JE. Conceptualizing and measuring life stress: Problems, principles, procedures, progress. Stress Medicine. 1990;6:209–216. [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological Bulletin. 1991;110(3):406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Owens M, Herbert J, Jones PB, Sahakian BJ, Wilkinson PO, Dunn VJ, Goodyer IM. Elevated morning cortisol is a stratified population-level biomarker for major depression in boys only with high depressive symptoms. Proceedings of the National Academy of Sciences. 2014;111(9):3638–3643. doi: 10.1073/pnas.1318786111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Skokie, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. Journal of consulting and clinical psychology. 1978;46(5):932. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schürmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences. 1999;64(18):1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2009;80(2):327–37. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Fuetsch M, Müller N, Höfler M, Lieb R, Wittchen HU. Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Archives of General Psychiatry. 2001;58(3):251–256. doi: 10.1001/archpsyc.58.3.251. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. Journal of the American Medical Association. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, DeRijk RH, Verhagen JC, van Dyck R, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Archives of General Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, DeRijk RH, Verhagen JC, van Dyck R, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosomatic Medicine. 2010;72(4):340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine. 2013;43(03):483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Yip KS. Understanding subjective depressive experiences of adolescents: Its implications to intervention. Paediatr. 2004;9:354–360. [Google Scholar]