Abstract
This study investigated the potential protective effects of ginseng on gamma-irradiation-induced oxidative stress and endothelial dysfunction in rats. Twenty four male albino rats were divided into four groups. In the control group, rats were administered vehicle by tube for 7 consecutive days. The second group was administered ginseng extract (100 mg/kg, by gavage) for 7 consecutive days. Animals in the third group were administered vehicle by tube for 7 consecutive days, then exposed to single dose gamma-irradiation (6 Gy). The Fourth group received ginseng extract for 7 consecutive days, one hour later rats were exposed to gamma-irradiation. Oral administration of ginseng extract prior to irradiation produced a significant protection which was evidenced by a significant reduction in serum creatine kinase (CPK) and lactate dehydrogenase (LDH) activities and asymmetric dimethylarginine (ADMA), urea and creatinine levels with significant increase in serum total nitrate/nitrite (NO(x)) level. Moreover, ginseng significantly increased cardiac and renal superoxide dismutase (SOD) and glutathione peroxidase (GSHPx) activities, and reduced glutathione (GSH) content, associated with a significant depletion in malondialdehyde (MDA) and NO(x) levels compared to irradiated group. This study suggests that ginseng may serve as a potential protective agent against gamma-irradiation-induced cardio-nephrotoxicity via enhancing the antioxidant activity and inhibition of endothelial dysfunction.
Keywords: ginseng, gamma-radiation, asymmetric dimethylarginine, nitric oxide, oxidative stress, rats
Introduction
Radiotherapy is frequently used as a part of cancer treatment to achieve tumor control. Although radiotherapy treatment has been widely used as an effective tool to kill tumor cells, it might produce harmful effects to surrounding healthy tissues (Ostrau et al., 2009[42]; Sezen et al., 2008[56]). It is well known that ionizing radiations induce oxidative stress on target tissues, mainly through the generation of reactive oxygen species (ROS) resulting in imbalance of the pro-oxidant and antioxidant in the cells, attack diverse cellular macromolecules such as DNA, lipids, and proteins, eventually inducing cell death (Boerma and Hauer-Jensen, 2011[7]; Lee et al., 2010[36]; Sathyasaikumar et al., 2007[52]).
Several studies have shown that irradiation plays an important role in the development of endothelial dysfunction; in particular, reduced bioavailability of nitric oxide (NO) (Soucy et al., 2010[59]). Asymmetric dimethylarginine (ADMA) is widely recognized as the major endogenous inhibitor of NO-synthase and is considered an emerging cardiovascular risk factor (Kiani et al., 2007[23]). Plasma concentration of ADMA is markedly increased in patients with chronic renal failure, and in a variety of cardiovascular diseases and moderately increased in patients with many other diseases including hyperlipidemia, diabetes mellitus, arterial hypertension, hyperhomocysteinemia and heart failure, suggesting that ADMA is an early marker of atherosclerosis vascular disease (Beltowski and Kêdra, 2006[4]).
Ginseng is one of the most highly valued natural dietary supplements (Yi et al., 2009[73]; Wu et al., 2011[71]). The term ginseng refers to the dried root of several species in the genus Panax of the Araliaceae family (Wang et al., 2007[67]) including two commonly used species, i.e., Panax ginseng C. A. Meyer (Asian ginseng) and Panax quinquefolius L (North American ginseng) (Lee and Lau, 2011[31]). Ginseng contains many physiologically important constituents including saponin (ginsenosides), polysaccharides, peptides, polyacetylenes, alkaloids, nitrogen-containing compounds, fatty acids and phenolic compounds (Attele et al., 1999[2]; Choi, 2008[11]; Lee et al., 2010[33]). Ginseng has drawn attention worldwide for its invaluable medicinal potential including antidiabetic, anticarcinogenic (Xie et al., 2005[72]; Wang et al., 2007[68]), analgesic, anti-pyretic, antistress, and antifatigue effects (Yi et al., 2009[73]; Lee et al., 2005[34]), as well as enhancement of immune-modulating capabilities (Hwanga et al., 2011[19]). These multifold bioactive medicinal properties of ginseng have been closely linked to its antioxidative effects (Kang et al., 2007[22]; Wang et al., 2007[67]). In addition, ginseng and its partially purified constituents have potential radioprotective properties (Lee et al., 2006[32]; Syaifudin et al., 2008[64]).
Therefore, the purpose of this study was to investigate the protective effect of ginseng against heart and kidney damage induced by gamma-radiation in rats.
Materials and Methods
Chemicals
Panax ginseng was purchased from Eipico, Egypt. It was suspended in 1 % carboxy methyl cellulose (CMC) in water and administrated orally to rats at a dose of 100 mg/kg body weight for 7 consecutive days (Gadkariem et al., 2010[13]).
Animals
Male Wistar rats (weighing 120-150 g) were obtained from the animal farm of the Egyptian Holding Company for Biological Products and Vaccines, Egypt. Upon arrival, the animals were allowed to acclimatize for 1 week before starting the experiment. Animals were kept under standard conditions and were allowed free access to a standard requirement diet and water ad libitum. Animals were kept under a controlled lighting condition (light: dark, 13 h-11 h). The animals' treatment protocol was approved by the Animal Care Committee of the National Center for Radiation Research and Technology (NCRRT), Cairo, Egypt.
Irradiation
Whole-body gamma-irradiation was performed at the National Centre for Radiation Research and Technology (NCRRT), Atomic Energy Authority, Cairo, Egypt, using (137 cesium) Gamma Cell-40 biological irradiator. Animals were irradiated at an acute single dose level of 6 Gy delivered at a dose rate of 0.012 Gy/s.
Experimental design
Male albino rats were divided into four groups, 6 rats in each. In the control group, rats were administered vehicle (0.5 ml of 1 % CMC suspension in water) by gavage for 7 consecutive days. The second group was administered ginseng (100 mg/kg, by gavage) for 7 consecutive days. Animals in the third group were administered vehicle by gavage for 7 consecutive days, then exposed to single dose γ-irradiation (6 Gy). The Fourth group received ginseng (100 mg/kg, by gavage) for 7 consecutive days, one hour later rats were exposed to single dose γ-irradiation (6 Gy) (Mansour and Hafez, 2012[38]).
Biochemical assays
Twenty-four hours after the last dose of the specific treatment, animals were anesthetized with ether, and blood samples were obtained by heart puncture and serum was separated by centrifugation (Sorvall TC centrifuge, Hamburg, Germany) at 750 g at room temperature for 10 min. Serum urea nitrogen, creatinine were determined according to the methods of Hallet and Cook (1971[15]) and Bonsenes and Taussky (1945[8]), respectively. Serum creatine phosphokinase (CPK) and Lactate Dehydrogenase were determined according to the methods of Swanson and Wilkinson (1972[63]) and IFCC (1980[21]) respectively. Serum total nitrate/nitrite (NO(x)) was measured as stable end product, nitrite, according to the method of Miranda et al. (2001[41]). ADMA was estimated using a standard enzyme linked immunosorbent assay (ELISA) method according to the manufacturer's instructions (Immundiagnostik AG, Bensheim/Germany).
Hearts and kidneys were quickly excised, washed with saline, blotted with a piece of filter paper and homogenized in ice-cold 0.15 MTris-KCl buffer (pH 7.4) to yield a 20 % (w/v) homogenate using a Branson sonifier (250, VWR Scientific, Danbury, CT, USA). The homogenates were used for the determination of malondialdehyde (MDA) level, glutathione peroxidase (GSHPx) and superoxide dismutase (SOD) activities, total glutathione (GSH) content, and total nitrate/nitrite (NO(x)). The homogenates were centrifuged at 800 g for 5 min at 4 ºC to separate the nuclear debris. The supernatant so obtained was centrifuged (Eppendorf AG, centrifuge 5804R, Hamburg, Germany) at 15000 g for 30 min at 4 ºC to get the post mitochondrial supernatant which was used to assay superoxide dismutase (SOD) activity.
Reduced glutathione (GSH) and malondialdehyde (MDA) levels in heart and kidney homogenates were determined spectrophotometrically using the methods of Ellman (1959[12]) and Buege and Aust (1978[9]), respectively. Total nitrate/nitrite (NO(x)) was measured as the stable end product, nitrite, according to the method of Miranda et al. (2001[41]). The activities of GSHPx and SOD were determined according to the methods of Lawrence and Burk (1976[30]) and Minami and Yoshikawa (1979[40]), respectively.
Statistical analysis
Results were expressed as mean ± SEM. The intergroup variation was measured by one way analysis of variance (ANOVA) followed by Tukey's Multiple comparison test. Statistical significance was considered at p < 0.05.
Results
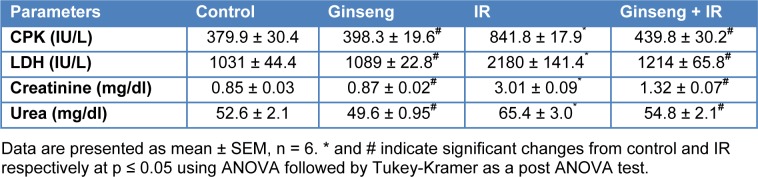
Table 1(Tab. 1) shows the effects of ginseng, irradiation and their combination on serum creatine phosphokinase (CPK), lactate dehydrogenase (LDH), creatinine and urea. Gamma-irradiation (6 Gy) induced a significant increase in CPK and LDH activities and significant increase in the levels of serum urea nitrogen and serum creatinine compared to control (P < 0.001). Administration of ginseng for 7 consecutive days before irradiation significantly reduced the activities of CPK and LDH, and the levels of urea and creatinine in serum (P < 0.001) compared to the irradiated group (Table 1(Tab. 1)).
Table 1. Effect of ginseng, irradiation (IR, 6 Gy) and their combination on serum creatine phosphokinase (CPK) and lactate dehydrogenase (LDH) activities, creatinine and urea levels.
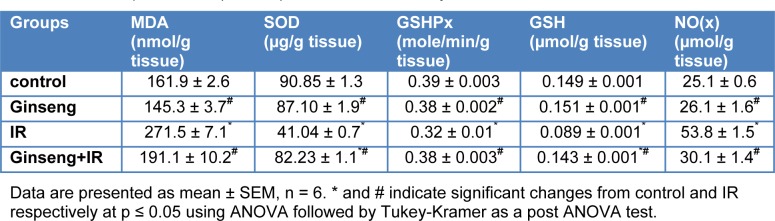
Table 2(Tab. 2) shows the effects of ginseng, gamma-irradiation and their combination on levels of GSH, MDA, and NO(x), the activity of SOD and GSHPx in kidney tissues. Gamma-irradiation exposure resulted in significant (54.9 %, 18 % and 40.3 %) decrease in SOD and GSHPx activities and GSH content and significant (67.7 % and 114.3 %) increase in MDA and NO(x), respectively, as compared to the control group. Treatment with ginseng for 7 consecutive days prior to irradiation resulted in a significant (100.4 %, 18.8 % and 60.7 %) increase in the activities of SOD and GSHPx and in GSH content, respectively, as compared with the irradiated group, and significant (29.6 % and 44.1 %) decrease in MDA and NO(x) levels ,respectively, compared to the irradiated group.
Table 2. Effect of ginseng, irradiation (IR, 6 Gy) and their combination on the levels of malondialdehyde (MDA), total nitrate/nitrite (NO(x)) and reduced glutathione (GSH), Superoxide dismutase (SOD) and Glutathione peroxidase (GSHPx) activities in rat kidney tissue.
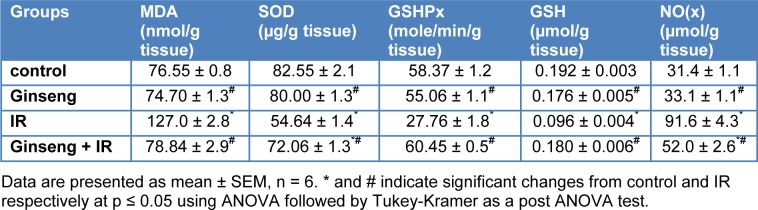
Table 3(Tab. 3) shows the effects of ginseng, gamma-irradiation and their combination on levels of GSH, MDA, and NO(x), the activity of SOD and GSHPx in cardiac tissues. Gamma-irradiation exposure resulted in significant (33.8 %, 52.4 % and 50 %) decrease in SOD and GSHPx activities and GSH content and significant (65.9 % and 191.7 %) increase in MDA and NO(x), respectively, as compared to the control group. Treatment with ginseng for 7 consecutive days prior to irradiation resulted in a significant (31.9 %, 117.8 % and 87.5 %) increase in the activities of SOD and GSHPx and in GSH content, respectively as compared with the irradiated group, and significant (37.9 % and 43.2 %) decrease in MDA and NO (x) levels, respectively, compared to the irradiated group.
Table 3. Effect of ginseng, irradiation (IR, 6 Gy) and their combination on the levels of malondialdehyde (MDA), total nitrate/nitrite (NO(x)) and reduced glutathione (GSH), Superoxide dismutase (SOD) and Glutathione peroxidase (GSHPx) activities in rat cardiac tissue.
Figures 1(Fig. 1) and 2(Fig. 2) show the effects of ginseng, irradiation and their combination on serum NO(x) and ADMA. Gamma-irradiation exposure resulted in a significant increase in the level of serum ADMA and significant decrease in serum NO(x) level compared to control (P < 0.001). Administration of ginseng for 7 consecutive days before irradiation significantly reduced the level of serum ADMA and the increase in serum NO(x) level (P < 0.001) compared to the irradiated group.
Figure 1. Effect of ginseng, irradiation (IR, 6 Gy) and their combination on serum total nitrate/nitrite (NO(x)).
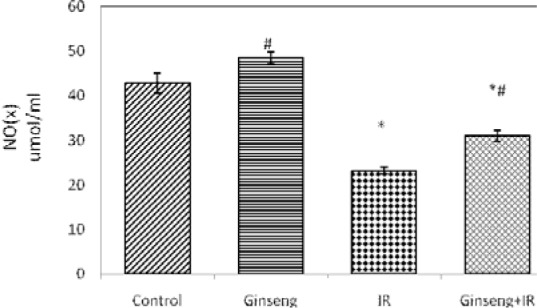
Data are presented as mean ± SEM, n = 6.
* and # indicate significant changes from control and IR respectively at p ≤ 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
Figure 2. Effect of ginseng, irradiation (IR, 6 Gy) and their combination on serum asymmetric dimethylarginine (ADMA).
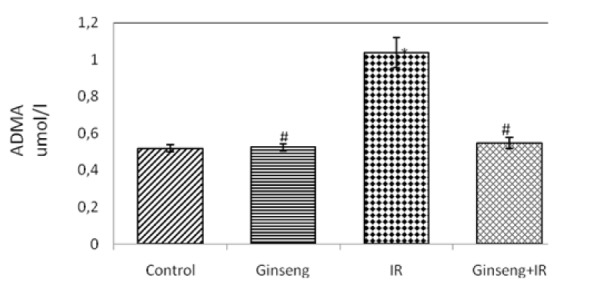
Data are presented as mean ± SEM, n = 6.
* and # indicate significant changes from control and IR respectively at p ≤ 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
Discussion
Ionizing radiation is known to induce oxidative stress through generation of ROS in an imbalance in pro-oxidant, antioxidant status in the cells (Bhosle et al., 2005[5]). In the present study, Gamma-irradiation caused a marked increase in serum activities of LDH and CPK, levels of creatinine, urea and ADMA in parallel with a significant decrease in NO(x) level. These data agree with that reported in previous studies, which reported that IRR caused a significant increase in CPK and LDH activities (Sridharan and Shyamaladevi, 2002[61]) and significant increase in urea and creatinine (Barakat et al., 2011[3]). The excessive production of free radicals and lipid peroxides might have caused the leakage of cytosolic enzymes such as lactate dehydrogenase, creatine kinase and phosphatases. Also, it could induce lipid peroxidation of cell membranes structure by oxygen derived free radicals leading to ionic leakage through cellular membranes and excessive calcium influx with ensuring cellular dysfunction and death from calcium overload (Ramadan et al., 1997[47]). Increase in serum urea was due to increase in glutamate de-hydrogenase enzyme as a result of irradiation and this may increase carbamoyl phosphate synthetase activity leading to increase in urea concentration (Ramadan et al., 2001[48]).
Treatment with ginseng for 7 consecutive days prior to irradiation ameliorated the activites of serum CPK and LDH and the levels of serum creatinine and urea. This effect might be related to the antioxidative properties of ginseng, which protect the outer membrane of mammalian cells (Block and Mead, 2003[6]). The antioxidative ability of ginseng is closely related to its ginsenoside content. Ginsenosides have the ability to intercalate into the plasma membrane, change its fluidity, and inhibit lipid peroxidation by chelating transition metals and scavenging ROS (Kang et al., 2007[22]), ginsenosides thus affect membrane function, eliciting cellular responses to cytotoxic stresses (Attele, 1999[2]).
In the present study, the γ-irradiated rats showed a significant increase in serum ADMA concomitantly with a significant decrease in NO(x) level. The effect is probably mediated by oxidative stress (Maas et al., 2007[37]). In agreement with our results, previous studies of Schnabel et al. (2005[53]), Busch et al. (2006[10]), Ueda et al. (2007[66]) and Wilcox (2012[70]) have reported elevated ADMA levels and decreased NO(x) levels in states of cardiovascular diseases and chronic kidney disease in human and rat and also in response of endothelial cells to ionizing radiation (Lanza et al., 2007[29]). Elevated levels of ADMA inhibit NO synthesis and therefore impair endothelial function (Sibal et al., 2010[57]). Reduction of NO(x) levels might be due to both decreased production and increased consumption, with possible endothelial dysfunction and vascular impairment (Soloviev et al., 2003[58]).
Consistent with previous studies (Kim and Lee, 2010[25]; Pan et al., 2012[43]), the present study showed that the endothelial NO(x) and ADMA levels were ameliorated by the administration of ginseng. Pan et al. (2012[43]) reported that, ginseng reduced ROS production and increased NO(x) levels, thus ameliorating endothelial dysfunction. Panax ginseng is known to enhance the release of nitric oxide (NO) from endothelial cells of the rat aorta and kidney, and to protect the heart from injury via up-regulation of endothelial NO synthase (eNOS) expression (Wu et al., 2011[71]; Razavi et al., 2005[50]; Hare and Stamler, 2005[18]; Han and Kim, 1996[16]), resulting in Ca(2+) channel inhibition, activation of cardiac potassium channels and protection against ischemia-reperfusion injury (Wang et al., 2008[69]; Szelid et al., 2010[65]) and this protection is caused by reducing oxidative stress (Kim and Lee, 2010[25]). These findings suggest that some of the observed effects of ginseng are possibly mediated through its antioxidant property.
Consistent with previous studies (Mansour and Hafez, 2012[38]; Pradeep et al., 2012[45]), the present study showed a significant depletion in the antioxidant system accompanied by enhancement of lipid peroxides and NO(x) levels in cardiac and renal tissues after whole body gamma-irradiation. Ionizing radiation is known to induce oxidative stress through generation of ROS in an imbalance in pro-oxidant, antioxidant status in the cells (Bhosle et al., 2005[5]). The increase in lipid peroxidation levels in γ-irradiated rats might be due to the interaction of free radicals with polyunsaturated fatty acids in the phospholipids portion of cellular membranes (Prasad et al., 2005[46]; Spitz et al., 2004[60]). The decrease in the activities of SOD and GSHPx and the decreased level of GSH might be due to their utilization by the enhanced production of ROS, which interacts with the enzyme molecules causing their denaturation and partial inactivation (Kregel and Zhang, 2007[27]; Maurya et al., 2006[39]). Under normal conditions the inherent defense system, including glutathione and the antioxidant enzymes, protects against oxidative damage. GSH, a well-known antioxidant, provides major protection in oxidative injury by participating in the cellular system of defense against oxidative damage (Sener et al., 2006[55]). It was reported that tissue GSH levels and the activities of glutathione reductase and glutathione peroxidase, which are critical constituents of GSH redox cycle, were significantly reduced due to oxidative stress, permitting enhanced free radical-induced tissue damage (Reiter et al., 2001[51]).
Our results show that whole body gamma-irradiation of rats at 6 Gy enhanced the formation of cardiac and renal NO(x). Similar results have been reported by Gorbunov et al. (2000[14]). Gamma-irradiation may enhance endogenous NO biosynthesis in liver, intestine, lung, kidney, brain, spleen or heart of the animals, presumably by facilitating the entry of Ca2+ ions into the membrane as well as the cytosol of NO-producing cells through irradiation-induced membrane lesions. The enhancement of NO production following exposure to a high dose (6 Gy) of gamma rays was attributed to high levels of expression of the inducible nitric oxide synthase (Ibuki and Goto, 1997[20]).
Consistent with previous studies (Abdel-Wahhab and Ahmed, 2004[1]; Kim et al., 2011[24]; Park et al., 2011[44]; Ramesh et al., 2012[49]), administration of ginseng markedly elevated the levels of antioxidant enzymes, indicating the antioxidant potential of the ginseng. This might be due to metabolized ginsenosides, which protect the outer membrane of mammalian cells (Block and Mead, 2003[6]; Lee et al., 2009[35]), scavenge free radicals, restore the GSH level and inhibit NO production (Zhu et al., 2009[75]; Zhang et al., 2010[74]). Panax ginseng extract has been shown to inhibit lipid peroxidation through transition metal chelation and scavenging of hydroxyl and superoxide radicals (Kitts et al., 2000[26]). It has been also reported that Panax ginseng administration increased the activity of the antioxidant enzymes SOD and GSHPx in rats (Han et al., 2005[17]; Sena et al., 2012[54]; Sun, 2011[62]).
Kumar et al. (2003[28]) found that administration of panax ginseng root extract before irradiation significantly decreased lipid peroxidation levels and reduced the radiation damage in mice testes.
The results from the present investigation indicate that ginseng pretreatment protects against radiation damage by inhibiting radiation-induced oxidative stress and endothelial dysfunction by decreasing serum ADMA, increasing serum NO(x) and ameliorating the antioxidant system in cardiac and renal tissues.
References
- 1.Abdel-Wahhab MA, Ahmed HH. Protective effect of Korean panax ginseng against chromium VI toxicity and free radicals generation in rats. J Ginseng Res. 2004;28:11–17. [Google Scholar]
- 2.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology. Biochem Pharmacol. 1999;58:168593. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 3.Barakat IAH, Abbas OA, Ayad S, Hassan AM. Evaluation of radioprotective effects of wheat germ oil in male rats. J Am Sci. 2011;7:664–673. [Google Scholar]
- 4.Beltowski J, Kdra A. Asymmetric dimethylarginine (ADMA) as a target for pharmacotherapy. Pharmacol Rep. 2006;59:159–178. [PubMed] [Google Scholar]
- 5.Bhosle SM, Huilgol NG, Mishra KP. Enhancement of radiation-induced oxidative stress and cytotoxicity in tumor cells by ellagic acid. Clin Chim Acta. 2005;359:89–100. doi: 10.1016/j.cccn.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Block KI, Mead MN. Immune system effects of Echinacea, ginseng, and astragalus: A review. Interg Cancer Ther. 2003;2:247– 67. doi: 10.1177/1534735403256419. [DOI] [PubMed] [Google Scholar]
- 7.Boerma M, Hauer-Jensen M. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract. 2011;2011:1–8. doi: 10.4061/2011/858262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonsenes RW, Taussky HN. On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem. 1945;158:587–591. [Google Scholar]
- 9.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 10.Busch M, Fleck C, Wolf G, Stein G. Asymmetrical (ADMA) and symmetrical dimethylarginine (SDMA) as potential risk factors for cardiovascular and renal outcome in chronic kidney disease - possible candidates for paradoxical epidemiology? Amino Acids. 2006;30:225–232. doi: 10.1007/s00726-005-0268-8. [DOI] [PubMed] [Google Scholar]
- 11.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:110918. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;17:214–226. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 13.Gadkariem EA, Al-Ashban RA, Babikir LB, Al-Joher HI. Toxicity study of Korean ginseng herbal medicine. Res J Pharmacol. 2010;4(4):86–90. [Google Scholar]
- 14.Gorbunov NV, Pogue-Geile KL, Epperly MW, Bigbee WL, Draviam R, Day BW, et al. Activation of the nitric oxide synthase 2 pathway in the response of bone marrow stromal cells to high doses of ionizing radiation. Radiat Res. 2000;154:73–86. doi: 10.1667/0033-7587(2000)154[0073:aotnos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Hallet CJ, Cook JG. Reduced nicotinamide adenine dinucleotide-coupled reaction for emergency blood urea estimation. Clin Chim Acta. 1971;35:33–37. doi: 10.1016/0009-8981(71)90289-0. [DOI] [PubMed] [Google Scholar]
- 16.Han SW, Kim H. Ginsenosides stimulate endogenous production of NO in rat kidney. Int J Biochem Cell Biol. 1996;28:573–580. doi: 10.1016/1357-2725(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Son SJ, Akhalaia M, Platonov A, Son HJ, Lee KH, et al. Modulation of radiation-induced disturbances of antioxidant defense systems by ginsan. Evid based Complement Alternat Med. 2005;2:52936. doi: 10.1093/ecam/neh123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:50917. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwanga I, Ahna G, Parka E, Haa D, Songb J, Jeea Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol Lett. 2011;138:16978. doi: 10.1016/j.imlet.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Ibuki Y, Goto R. Enhancement of NO production from resident peritoneal macrophages by in vitro gamma-irradiation and its relationship to reactive oxygen intermediates. Free Radic Biol Med. 1997;22:1029–1035. doi: 10.1016/s0891-5849(96)00500-x. [DOI] [PubMed] [Google Scholar]
- 21.IFCC. Measurement of lactate dehydrogenase in serum. J Clin Chem Clin Biochem. 1980;18:521. [Google Scholar]
- 22.Kang KS, Kim HY, Baek SH, Yoo HH, Park JH, Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 23.Kiani AN, Mahoney JA, Petri M. Asymmetric dimethylarginine is a marker of poor prognosis and coronary calcium in systemic lupus erythematosus. J Rheumatol. 2007;34:1502–1505. [PubMed] [Google Scholar]
- 24.Kim HJ, Lee SG, Chae IG, Kim MJ, Im NK, Yu MH, et al. Antioxidant effects of fermented red ginseng extracts in streptozotocin-induced diabetic rats. J Ginseng Res. 2011;35:12937. doi: 10.5142/jgr.2011.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TH, Lee SM. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48:151620. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Kitts D, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 27.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 28.Kumar M, Sharma MK, Saxena PS, Kumar A. Radioprotective effect of panax ginseng on the phosphatases and lipid peroxidation level in testes of Swiss albino mice. Biol Pharm Bull. 2003;26:30812. doi: 10.1248/bpb.26.308. [DOI] [PubMed] [Google Scholar]
- 29.Lanza V, Fadda P, Iannone C, Negri R. Low-dose ionizing radiation stimulates transcription and production of endothelin by human vein endothelial cells. Radiat Res. 2007;168:193–198. doi: 10.1667/RR0780.1. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee DCW, Lau ASY. Effects of Panax ginseng on tumor necrosis factor-?-mediated inflammation: A mini-review. Molecules. 2011;16:2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HJ, Kim SR, Kim JC, Kang CM, Lee YS, Jo SK, et al. In vivo radioprotective effect of Panax ginseng C.A. Meyer and identification of active ginsenosides. Phytother Res. 2006;20:392–395. doi: 10.1002/ptr.1867. [DOI] [PubMed] [Google Scholar]
- 33.Lee SY, Kim YK, Park N, Kim CS, Lee CY, Park SU. Chemical constituents and biological activities of the berry of Panax ginseng. J Med Plants Res. 2010;5:34953. [Google Scholar]
- 34.Lee TK, Johnke RM, Allison RR, OBrien KF, Dobbs LJ. The radioprotective potential of ginseng. Mutagenesis. 2005;20:237– 43. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 35.Lee TK, OBrien KF, Wang W, Chao Sheng C, Wang T, Johnke RM, et al. American ginseng modifies 137Cs-induced DNA damage and oxidative stress in human lymphocytes. Open Nuclear Med J. 2009;1:1–8. doi: 10.2174/1876388X00901010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee TK, OBrien KF, Wang W, Johnke RM, Sheng C, Benhabib SM, et al. Radioprotective effect of American ginseng on human lymphocytes at 90 minutes postirradiation: A study of 40 cases. J Altern Complement Med. 2010;16:5617. doi: 10.1089/acm.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maas R, Schulze F, Baumert J, Lowel H, Hamraz K, Schwedhelm E, et al. Asymmetric dimethylarginine, smoking, and risk of coronary heart disease in apparently healthy men: prospective analysis from the population-based monitoring of trends and determinants in cardiovascular disease. Clin Chem. 2007;53:693–701. doi: 10.1373/clinchem.2006.081893. [DOI] [PubMed] [Google Scholar]
- 38.Mansour HH, Hafez HF. Protective effect of Withania somnifera against radiation-induced hepatotoxicity in rats. Ecotoxicol Environ Saf. 2012;80:14–19. doi: 10.1016/j.ecoenv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Maurya DK, Devasagayam TPA, Nair CKK. Some novel approaches for radioprotection and the beneficial effect of natural products. Indian J Exp Biol. 2006;44:93114. [PubMed] [Google Scholar]
- 40.Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta. 1979;92:337–342. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 41.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 42.Ostrau C, Hlsenbeck J, Herzog M, Schad A, Torzewski M, Lackner KJ, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009;92:4929. doi: 10.1016/j.radonc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Pan C, Huo Y, An X, Singh G, Chen M, Yang Z, et al. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vasc Pharmacol. 2012;56:150–158. doi: 10.1016/j.vph.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Park E, Hwang I, Song J, Jee Y. Acidic polysaccharide of Panax ginseng as a defense against small intestinal damage by whole-body gamma irradiation of mice. Acta Histochem. 2011;113:1923. doi: 10.1016/j.acthis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Pradeep K, Ko KC, Choi MH, Kang JA, Chung YJ, Park SH. Protective effect of hesperidin, a citrus flavanoglycone, against ?-radiation-induced tissue damage in Sprague-Dawley rats. J Med Food. 2012;15:419–427. doi: 10.1089/jmf.2011.1737. [DOI] [PubMed] [Google Scholar]
- 46.Prasad NR, Menon VP, Vasudev V, Pugalendi KV. Radioprotective effect of sesamol on ?-radiation induced DNA damage, lipid peroxidation and antioxidants levels in cultured human lymphocytes. Toxicology. 2005;209:225–235. doi: 10.1016/j.tox.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Ramadan LA, Moustafa AMA, El-Sayed EM. A possible protecting activity of diltiazem against irradiation hazards on the cardiac muscle. Az J Pharm Sci. 1997;19:1–8. [Google Scholar]
- 48.Ramadan LA, Shouman SA, Sayed-Ahmed MM, El-Habit OH. Modulation of radiation-induced organs toxicity by cremophor-el in experimental animals. Pharmacol Res. 2001;43:185–191. doi: 10.1006/phrs.2000.0763. [DOI] [PubMed] [Google Scholar]
- 49.Ramesh T, Kim SW, Sung JH, Hwang SY, Sohn SH, Yoo SK, et al. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol. 2012;47:7784. doi: 10.1016/j.exger.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Razavi HM, Hamilton JA, Feng Q. Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacol Ther. 2005;147:160–162. doi: 10.1016/j.pharmthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species. A review of the evidence. Cell Biochem Biophys. 2001;34:23756. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 52.Sathyasaikumar KV, Swapna I, Reddy PVB, Murthy ChRK, Dutta Gupta A, Senthilkumaran B, et al. Fulminant hepatic failure in rats induces oxidative stress differentially in cerebral cortex, cerebellum and pons medulla. Neurochem Res. 2007;32:51724. doi: 10.1007/s11064-006-9265-x. [DOI] [PubMed] [Google Scholar]
- 53.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97:e539. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 54.Sena S, Chena S, Fenga B, Wua Y, Luib E, Chakrabartia S. Preventive effects of North American ginseng (Panax quinquefolium) on diabetic nephropathy. Phytomedicine. 2012;19:494505. doi: 10.1016/j.phymed.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Sener G, Kabasakal L, Atasoy BM, Erzik C, Velioglugunc A, Cetinel S, et al. Ginkgo biloba extract protects against ionizing radiation-induced oxidative organ damage in rats. Pharmacol Res. 2006;53:24152. doi: 10.1016/j.phrs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Sezen O, Ertekin MV, Demircan B, Karslio?lu I, Erdo?an F, Koer I, et al. Vitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced brain and retinal damages. Neurosurg Rev. 2008;31:20513. doi: 10.1007/s10143-007-0118-0. [DOI] [PubMed] [Google Scholar]
- 57.Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010;6:82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soloviev AI, Tishkin SM, Parshikov AV, Ivanova IV, Goncharov EV, Gurney AM. Mechanisms of endothelial dysfunction after ionized radiation: selective impairment of the nitric oxide component of endothelium dependent vasodilation. Br J Pharmacol. 2003;138:837–844. doi: 10.1038/sj.bjp.0705079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soucy KG, Lim HK, Attarzadeh DO, Santhanam L, Kim JH, Bhunia AK, et al. Dietary inhibition of xanthine oxidase attenuates radiation-induced endothelial dysfunction in rat aorta. J Appl Physiol. 2010;108:1250–1258. doi: 10.1152/japplphysiol.00946.2009. [DOI] [PubMed] [Google Scholar]
- 60.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metast Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 61.Sridharan S, Shyamaladevi CS. Protective effect of N-acetylcysteine against gamma ray induced damages in rats biochemical evaluations. Indian J Exp Biol. 2002;40:181–186. [PubMed] [Google Scholar]
- 62.Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. Meyer: An overview. Carbohydrate Polymers. 2011;85:4909. [Google Scholar]
- 63.Swanson JR, Wilkinson JH. Measurement of creatine kinase in serum. In: Cooper GR, editor. Standard method of clinical chemistry. Vol. 7. New York: Academic Press; 1972. [Google Scholar]
- 64.Syaifudin M, Song JY, Lee YS, Kang CM. Radio protective effects of ginseng extract in gamma-rays induced chromosomal damages of human lymphocyte. Atom Indonesia. 2008;34(1):45–58. [Google Scholar]
- 65.Szelid Z, Pokreisz P, Liu X, Vermeersch P, Marsboom G, Gillijns H, et al. Cardioselective nitric oxide synthase 3 gene transfer protects against myocardial reperfusion injury. Basic Res Cardiol. 2010;105:16979. doi: 10.1007/s00395-009-0077-4. [DOI] [PubMed] [Google Scholar]
- 66.Ueda S, Yamagishi S, Matsumoto Y, Fukami K, Okuda S. Asymmetric dimethylarginine (ADMA) is a novel emerging risk factor for cardiovascular disease and the development of renal injury in chronic kidney disease. Clin Exp Nephrol. 2007;11:115–121. doi: 10.1007/s10157-007-0471-x. [DOI] [PubMed] [Google Scholar]
- 67.Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: Active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, Zhao Y, Raybur ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z, Li M, Wu WK, Tan HM, Geng DF. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther. 2008;22:44352. doi: 10.1007/s10557-008-6129-4. [DOI] [PubMed] [Google Scholar]
- 70.Wilcox CS. Asymmetric dimethylarginine and reactive oxygen species. Unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59:375–381. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Y, Lu X, Xiang FL, Lui EM, Feng Q. North American ginseng protects the heart from ischemia and reperfusion injury via upregulation of endothelial nitric oxide synthase. Pharmacol Res. 2011;64:195–202. doi: 10.1016/j.phrs.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Xie JT, McHendale S, Yuan CS. Ginseng and diabetes. Am J Chin Med. 2005;33:397–404. doi: 10.1142/S0192415X05003004. [DOI] [PubMed] [Google Scholar]
- 73.Yi SW, Sull JW, Hong JS, Linton JA, Ohrr H. Association between ginseng intake and mortality: Kangwha cohort study. J Altern Complement Med. 2009;15:9218. doi: 10.1089/acm.2008.0296. [DOI] [PubMed] [Google Scholar]
- 74.Zhang HA, Wang M, Zhou J, Yao QY, Ma JM, Jiang CL. Protective effect of ginsenoside against acute renal failure and expression of tyrosine hydroxylase in the locus coeruleus. Physiol Res. 2010;59:6170. doi: 10.33549/physiolres.931650. [DOI] [PubMed] [Google Scholar]
- 75.Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong ZY, et al. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem. 2009;108:11724. doi: 10.1002/jcb.22233. [DOI] [PubMed] [Google Scholar]