Abstract
BACKGROUND
Hereditary angioedema is characterized by recurrent attacks of angioedema of the skin, larynx, and gastrointestinal tract. Bradykinin is the key mediator of symptoms. Icatibant is a selective bradykinin B2 receptor antagonist.
METHODS
In two double-blind, randomized, multicenter trials, we evaluated the effect of icatibant in patients with hereditary angioedema presenting with cutaneous or abdominal attacks. In the For Angioedema Subcutaneous Treatment (FAST) 1 trial, patients received either icatibant or placebo; in FAST-2, patients received either icatibant or oral tranexamic acid, at a dose of 3 g daily for 2 days. Icatibant was given once, subcutaneously, at a dose of 30 mg. The primary end point was the median time to clinically significant relief of symptoms.
RESULTS
A total of 56 and 74 patients underwent randomization in the FAST-1 and FAST-2 trials, respectively. The primary end point was reached in 2.5 hours with icatibant versus 4.6 hours with placebo in the FAST-1 trial (P = 0.14) and in 2.0 hours with icatibant versus 12.0 hours with tranexamic acid in the FAST-2 trial (P<0.001). In the FAST-1 study, 3 recipients of icatibant and 13 recipients of placebo needed treatment with rescue medication. The median time to first improvement of symptoms, as assessed by patients and by investigators, was significantly shorter with icatibant in both trials. No icatibant-related serious adverse events were reported.
CONCLUSIONS
In patients with hereditary angioedema having acute attacks, we found a significant benefit of icatibant as compared with tranexamic acid in one trial and a nonsignificant benefit of icatibant as compared with placebo in the other trial with regard to the primary end point. The early use of rescue medication may have obscured the benefit of icatibant in the placebo trial. (Funded by Jerini; ClinicalTrials.gov numbers, NCT00097695 and NCT00500656.)
Hereditary angioedema is an autosomal dominant disorder caused by a deficiency of C1 esterase inhibitor, which has a regulatory role in the classic complement pathway and in the coagulation, fibrinolytic, and kallikrein– kinin (contact-system) cascades. Reduced activity of C1 esterase inhibitor may result in an elevated plasma level of bradykinin,1,2 the key mediator of symptoms in hereditary angioedema.3,4
Patients with hereditary angioedema present with acute attacks of subcutaneous and submucosal edema that can affect the upper airways, face, extremities, genitals, and gastrointestinal tract.5–7 Pharyngolaryngeal edema, which is potentially life-threatening because of the risk of upper-airway obstruction, can also occur.8
Current treatment options for hereditary angioedema are limited. Tranexamic acid, an oral anti-fibrinolytic agent, reportedly improves symptoms during acute attacks,9 although its efficacy has not been proved in controlled studies. Attenuated androgens can reduce the number and severity of attacks when used prophylactically but have important adverse effects and are ineffective during acute attacks.10–12 Replacement therapy with intravenous C1 esterase–inhibitor concentrate reverses acute attacks.13,14 However, formulations of this agent are registered in only a few countries.
Icatibant is a selective bradykinin B2 receptor antagonist15,16 with, like bradykinin itself, an affinity for the B2 receptor. It does not interact with bradykinin B1 receptors or other peptide receptors.17 Icatibant reverses increased vascular permeability in C1 esterase inhibitor–knockout mice,18 inhibits bradykinin-induced vasodilation in humans,4,19 and, in a phase 1 study, showed dose-and time-dependent inhibition of bradykinin-induced effects in vivo.20 In a phase 2 study, symptoms improved significantly after open-label treatment with icatibant in 15 patients with hereditary angioedema having acute cutaneous or abdominal attacks.1
We conducted two phase 3 studies of icatibant treatment for acute attacks of angioedema in adults with hereditary angioedema due to deficiency of the C1 esterase inhibitor. Results from the controlled phase of both studies are presented here.
METHODS
STUDY DESIGN
The For Angioedema Subcutaneous Treatment (FAST)-1 and FAST-2 trials were double-blind, randomized, prospective studies. FAST-1 was placebo-controlled. In FAST-2, icatibant was compared with tranexamic acid. Both were multicenter trials (see Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Each study was approved by the independent ethics committee at each center and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and current regulatory requirements. The complete study protocols and statistical-analysis plans are available at NEJM.org.
Both trials were designed and sponsored by Jerini. Monitoring and data collection were supervised for FAST-1 by Hesperion and Parexel International and for FAST-2 by Hesperion and Amantec. Data management and statistical analysis in both trials were performed by Hesperion. The academic authors vouch for the accuracy and completeness of the data and all analyses, as well as the fidelity of the reported information to the protocols.
PATIENTS
Before enrollment, inclusion and exclusion criteria were assessed in all patients, and all patients gave written informed consent. In both trials, the eligibility criteria included an age of 18 years or older and a documented diagnosis of hereditary angioedema type I (from an antigenic deficiency of the C1 esterase inhibitor) or type II (from a functional deficiency of the C1 esterase inhibitor). Diagnosis was confirmed by a review of the medical history and a finding of levels of functional or antigenic C1 esterase inhibitor of less than half the normal levels. For patients receiving androgens, historical laboratory values were used to confirm the diagnosis. The main exclusion criteria included a diagnosis of angioedema other than hereditary angioedema, serious concomitant illness, and pregnancy or lactation.
After completion of the screening visit, eligible patients were asked to return to the study site no later than 6 hours after an acute attack of angioedema became at least moderately severe, to undergo assessment before randomization. Patients were excluded if they had received pain medication for the current attack, C1 esterase inhibitor within 3 days before the attack, or tranexamic acid within a week before the attack or if they were receiving angiotensin-converting–enzyme inhibitors or were pregnant. Patients presenting with cutaneous or abdominal attacks were asked to evaluate the severity of three specific symptoms (cutaneous swelling, cutaneous pain, and abdominal pain) on a visual-analogue scale. For each symptom, patients drew a vertical line on a nongraduated 100-mm visual-analogue scale, according to severity (with 0 mm indicating no symptoms, and 100 mm indicating symptoms of the worst possible severity). Patients with a score on the visual-analogue scale of at least 30 mm for one or more of the three symptoms, and no exclusion criteria, were considered to have had an eligible attack.
STUDY TREATMENT
Patients with eligible attacks were randomly assigned (in a 1:1 ratio and after stratification, with the use of stochastic minimization, according to study center and the site of edema) to receive either subcutaneous icatibant, at a dose of 30 mg, or a matched subcutaneous placebo injection (in the FAST-1 trial) or oral tranexamic acid at a dose of 3 g daily for 2 days (in the FAST-2 trial). Blinding was maintained in the FAST-1 trial by means of the identical appearance of the placebo and in the FAST-2 trial by means of a double-dummy design (i.e., concomitant administration of placebo capsules or subcutaneous placebo injection). In the FAST-2 trial, patients received two further doses of oral medication (either tranexamic acid or placebo) on day 1, one at 6 hours and one at 12 hours after the initial study-drug administration, followed by three further doses, each 6 to 8 hours apart, on day 2.
In both trials, any patient having a subsequent attack of sufficient severity to necessitate treatment was included in an open-label extension phase and received icatibant. Patients with potentially life-threatening laryngeal angioedema were also treated with open-label icatibant.
Patients were hospitalized for up to 15 hours. Symptoms were assessed by patients, using the visual-analogue scale, at 30-minute intervals between 1 and 4 hours after administration of the study drug and also at 5, 6, 8, 10, and 12 to 15 hours after administration. Patients then assessed their symptoms three times a day, from day 2 to day 5 or until symptoms had subsided. Follow-up visits were scheduled for days 2 and 14, week 5, and week 24. Blood samples were obtained before study-drug administration and on days 1, 2, and 14, week 5, and week 24 after administration.
Concomitant medication for other conditions was permitted if it did not interfere with study objectives (see the Supplementary Appendix). Rescue therapy (i.e., with C1 esterase inhibitor concentrate, antiemetic agents, or opiates) for relief of any symptom was permitted but withheld for as long as possible (ideally until 8 or 9 hours after study-drug administration). Data for patients who needed rescue therapy were not censored or excluded from analyses.
END POINTS
Based on the highest score on the visual-analogue scale before study-drug administration, one of the three main symptoms (cutaneous swelling, cutaneous pain, or abdominal pain) was defined in each patient as the index symptom for purposes of assessing the primary end point. For attacks with a combination of these symptoms, abdominal pain was considered the index symptom.
The primary efficacy end point was the median time to clinically significant relief of the index symptom. Clinically significant symptom relief was defined as a minimum decrease in the score on the visual-analogue scale of 20 to 30 mm, depending on the initial symptom severity. Therefore, the decrease in severity necessary to achieve the primary end point was at least 30% (i.e., 30-mm decrease from a baseline score of 100 mm). This decrease had to have been sustained for three consecutive measurements on the visual-analogue scale, with the first measurement being the time point at which clinically significant relief was achieved.
Secondary efficacy end points included the median times to first symptom improvement according to the patient and according to the investigator; the median time to almost complete relief of symptoms (i.e., the end of an attack), defined as the time point at which the score on the visual-analogue scale was 0 to 10 mm for at least three consecutive measurements for all symptoms, and the proportion of patients reaching the median time to clinically significant relief of the index symptom within 4 hours after the start of the study drug. Patients receiving open-label icatibant for laryngeal edema also had their symptoms assessed after the start of study treatment, both by themselves and by an investigator (see the Supplementary Appendix).
Safety was evaluated by means of adverse-event reporting, documentation of local tolerability (i.e., injection-site reactions), measurement of vital signs, electrocardiography, clinical laboratory testing, urinalysis, and assessment of complement activation. For patients from whom at least one serum sample had been obtained after the start of the study drug, testing for anti-icatibant antibodies was performed with the use of enzyme-linked immunosorbent assays.
STATISTICAL ANALYSIS
On the basis of historical data for the patients, we estimated that the mean time to clinically significant relief of the index symptom for nontreated attacks would be 16.8 hours, with a variance of 44.8 hours. Using data from the same patients after treatment with icatibant in a phase 2 trial,1 we estimated a mean and variance of the time to clinically significant relief with icatibant of 1.9 hours and 1.4 hours, respectively. For the power calculation, variances of 4 hours for icatibant, 46 hours for tranexamic acid, and 64 hours for placebo were used. For the FAST-1 trial, 20 patients per group were needed to provide 80% power to detect a difference of 5.5 hours in the median time to clinically significant relief between the icatibant group and the placebo group in the FAST-1 trial with a two-sided type I error of 0.05. For the FAST-2 trial, 27 patients per group were needed to detect a difference in the median time to clinically significant relief of 4.0 hours between the icatibant group and the tranexamic acid group. After inflating the sample sizes by 20% to adjust for the use of a nonparametric Wilcoxon test, we calculated that we would need to enroll 25 and 33 patients per group for the FAST-1 trial and FAST-2 trial, respectively. The final sample sizes of 56 and 74 patients, respectively, accounted for a projected dropout rate of 10%.
The prespecified primary analysis was based on the intention-to-treat population, which included all patients who underwent randomization. Comparisons between groups were performed with the use of the prespecified Wilcoxon log-rank test with a two-sided significance level of 5%. The Wilcoxon test was used for the time-to-event analysis. For the primary end point, results based on the two tests were checked, verified, and found to be identical.
All secondary analyses were prespecified. All time-to-event data were evaluated as for the primary analysis. Fisher’s exact test, with 95% confidence intervals calculated for each group by means of the Clopper–Pearson method, was used to compare the percentage of patients with clinically significant relief of the index symptom at 4 hours after the start of the study drug. Two-sided 95% confidence intervals for the difference in proportions were calculated with the use of the Anderson–Hauck correction.
All reported P values are two-sided and were not adjusted for multiple testing. No interim analyses were performed. Demographic, safety, and tolerability results are reported descriptively.
RESULTS
PATIENTS
In the FAST-1 trial, 178 patients were screened and 64 enrolled (Fig. 2 in the Supplementary Appendix). Of these, 56 were randomly assigned to receive icatibant (27 patients) or placebo (29 patients). In the FAST-2 trial, 247 patients were screened and 77 enrolled (Fig. 3 in the Supplementary Appendix). Of these, 74 were randomly assigned to receive icatibant (36 patients) or tranexamic acid (38 patients). The baseline characteristics were similar between study treatment groups in both trials, although the sex ratios differed in the FAST-1 study (Table 1).
Table 1.
Baseline Characteristics of the Study Patients in the FAST-1 and FAST-2 Trials, According to Study Group.*
| Characteristic | FAST-1 | FAST-2 | ||
|---|---|---|---|---|
| Icatibant (N = 27) | Placebo (N = 29) | Icatibant (N = 36) | Tranexamic Acid (N = 38) | |
| Sex — no. (%) | ||||
| Male | 11 (41) | 8 (28) | 12 (33) | 15 (39) |
| Female | 16 (59) | 21 (72) | 24 (67) | 23 (61) |
| Age — yr | 34.8±9.8 | 34.9±11.4 | 40.4±13.6 | 41.9±12.4 |
| Weight — kg | 80.3±21.1 | 76.0±21.9 | 80.1±16.1 | 74.2±15.6 |
| Type of HAE attack — no. | ||||
| Cutaneous | 14 | 13 | 24 | 23 |
| Abdominal | 13 | 16 | 12 | 15 |
| No. of attacks in previous 6 mo — median (range) | ||||
| Cutaneous | 6 (1–44) | 6 (1–50) | 6 (1–24) | 5 (1–25) |
| Abdominal | 3 (1–24) | 5 (2–16) | 3 (1–15) | 3 (1–72) |
| Cutaneous and abdominal | 2 (1–40) | 5 (1–12) | 4 (1–24) | 2 (1–12) |
| Laryngeal | 1 (1–4) | 1 (1–9) | 2 (1–7) | 1 (1–6) |
Plus–minus values are means ±SD. HAE denotes hereditary angioedema.
END POINTS
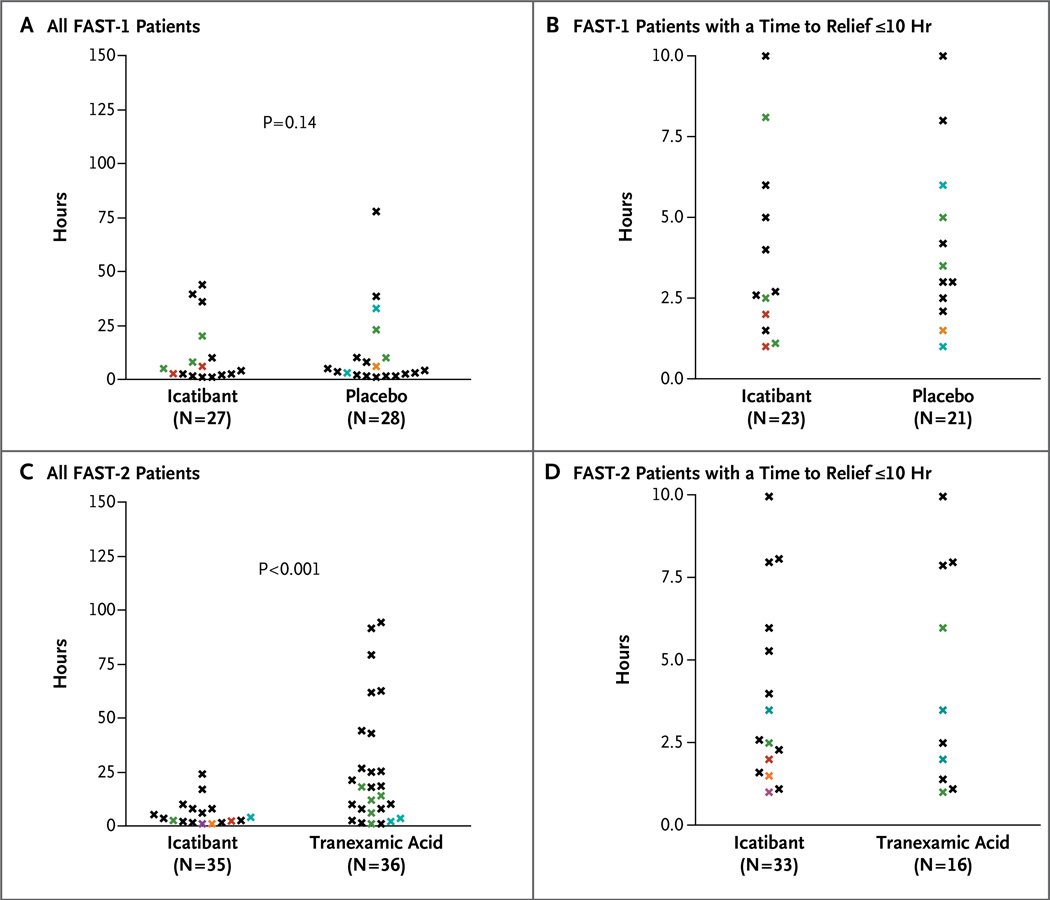
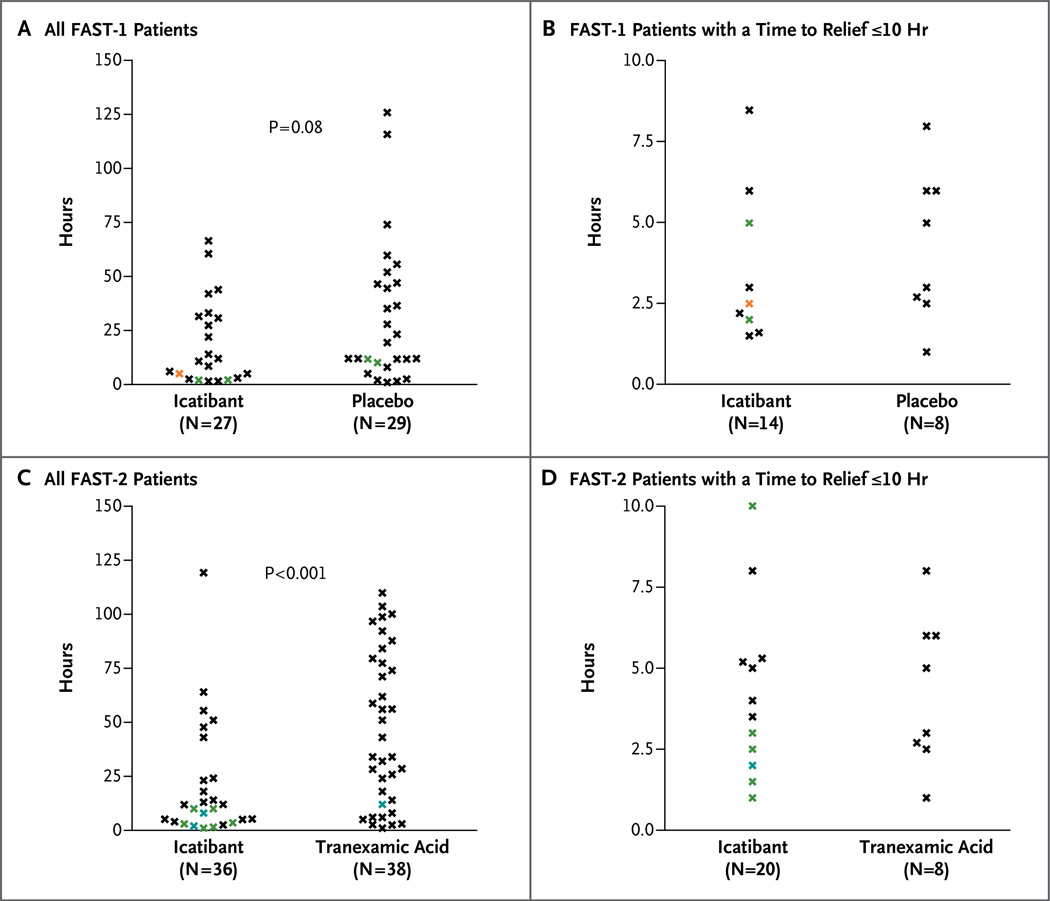
In FAST-1, the primary end point, the median time to clinically significant relief of the index symptom, was 2.5 hours in the icatibant group versus 4.6 hours in the placebo group (P = 0.14) (Table 2 and Fig. 1A). In FAST-2, the median time to clinically significant relief of the index symptom was 2.0 hours with icatibant versus 12.0 hours with tranexamic acid (P<0.001) (Table 2 and Fig. 1B). The median time to almost complete relief of symptoms was 8.5 hours with icatibant versus 19.4 hours with placebo (P = 0.08) in FAST-1 (Table 2 and Fig. 2A) and 10.0 hours with icatibant versus 51.0 hours with tranexamic acid (P<0.001) in FAST-2 (Table 2 and Fig. 2B). The percentage of patients with clinically significant relief of the index symptom at 4 hours after the start of study treatment was 67% in the icatibant group versus 46% in the placebo group in FAST-1 (P = 0.18) and 80% in the icatibant group versus 31% in the tranexamic acid group in FAST-2 (P<0.001) (Table 2).
Table 2.
Clinical Outcomes in the FAST-1 and FAST-2 Trials, According to Study Group.*
| Outcome | FAST-1 | FAST-2 | ||||
|---|---|---|---|---|---|---|
| Icatibant (N = 27) |
Placebo (N = 29) |
P Value | Icatibant (N = 36) |
Tranexamic Acid (N = 38) |
P Value | |
| Time to clinically significant relief of the index symptom (primary end point) — hr |
0.14 | <0.001 | ||||
| Median | 2.5 | 4.6 | 2.0 | 12.0 | ||
| IQR | 1.1–6.0 | 1.8–10.2 | 1.0–3.5 | 3.5–25.4 | ||
| Time to first symptom improvement — hr | ||||||
| According to patient | <0.001 | <0.001 | ||||
| Median | 0.8 | 16.9 | 0.8 | 7.9 | ||
| IQR | 0.5–2.0 | 3.2–NA | 0.4–1.4 | 1.1–NA | ||
| According to investigator | <0.001 | <0.001 | ||||
| Median | 1.0 | 5.7 | 1.5 | 6.9 | ||
| IQR | 0.8–2.0 | 2.0–11.2 | 0.7–3.0 | 4.0–13.8 | ||
| Time to almost complete relief of symptoms — hr | 0.08 | <0.001 | ||||
| Median | 8.5 | 19.4 | 10.0 | 51.0 | ||
| IQR | 2.5–31.5 | 10.2–55.7 | 2.8–23.2 | 12.0–79.5 | ||
| Clinically significant relief of the index symptom at 4 hr after start of study drug — % (95% CI) |
67 (46–84) | 46 (28–66)† | 0.18 | 80 (63–92)† | 31 (16–48)‡ | <0.001 |
NA denotes not available, CI confidence interval, and IQR interquartile range.
Data are missing for one patient.
Data are missing for two patients.
Figure 1. Time to Clinically Significant Relief of the Index Symptom in the FAST-1 and FAST-2 Trials, According to Study Group.
The time to clinically significant relief of the index symptom is shown for all FAST-1 patients (Panel A) and all FAST-2 patients (Panel C) with data, as well as patients in whom the time was 10 hours or less (Panels B and D for FAST-1 and FAST-2 patients, respectively). Black data points represent individual patients; colored data points represent multiple patients at the same time point: green, two patients; blue, three patients; orange, four patients; red, five patients; and purple, nine patients. In the FAST-2 trial, data were not available for one patient in the icatibant group and two patients in the tranexamic group whose score on the visual-analogue scale was less than 30 mm at baseline.
Figure 2. Time to Almost Complete Relief of Symptoms in the FAST-1 and FAST-2 Trials, According to Study Group.
The time to almost complete relief of symptoms is shown for all FAST-1 patients (Panel A) and all FAST-2 patients (Panel C) with data, as well as patients in whom the time was 10 hours or less (Panels B and D for FAST-1 and FAST-2 patients, respectively). Black data points represent individual patients; colored data points represent multiple patients at the same time point: green, two patients; blue, three patients; orange, four patients; red, five patients; and purple, nine patients.
The median time to first symptom improvement was significantly shorter with icatibant than with placebo in FAST-1, as assessed by the patient (0.8 vs. 16.9 hours, P<0.001) or the investigator (1.0 vs. 5.7 hours, P<0.001) (Table 2). Similarly, the median time was significantly shorter with icatibant than with tranexamic acid in FAST-2, as assessed by the patient (0.8 vs. 7.9 hours, P<0.001) or the investigator (1.5 vs. 6.9 hours, P<0.001) (Table 2).
POST HOC ANALYSES
Several post hoc analyses were undertaken in an effort to understand the differences between the two studies with respect to the primary end point. A composite score on the visual-analogue scale was calculated by taking the average of the three scores for the main symptoms — abdominal pain, cutaneous pain, and cutaneous swelling — and defining symptom relief as a 50% reduction from the baseline score. On the basis of the composite scores, the median time to symptom relief was 2.5 hours with icatibant versus 7.0 hours with placebo (P = 0.02) in FAST-1 and 2.0 hours with icatibant versus 15.0 hours with tranexamic acid (P<0.001) in FAST-2 (Fig. 4 in the Supplementary Appendix). Data were censored at 120 hours (the end of the observation period) for patients who took rescue medication before the onset of symptom relief. After censoring, analysis resulted in a time to relief of the index symptom of 2.5 hours with icatibant versus 9.0 hours with placebo (P = 0.02) in FAST-1 and 2.0 hours with icatibant versus 16.0 hours with tranexamic acid (P<0.001) in FAST-2 (Table 2 in the Supplementary Appendix).
Several additional post hoc analyses were performed. Most of these also yielded evidence of a benefit of icatibant in both trials, although in a few analyses the benefit was not significant (Table 3 in the Supplementary Appendix).
RESCUE MEDICATION
In the FAST-1 trial, rescue medication was administered within the first 12 hours in 3 of the 27 patients (11%) receiving icatibant, as compared with 13 of the 29 patients (45%) receiving placebo, and within the first 48 hours in 6 patients (22%) and 15 patients (52%), respectively. In the FAST-2 trial, rescue medication was administered within the first 12 hours in 0 of the 36 patients receiving icatibant, as compared with 5 of the 38 patients (13%) receiving tranexamic acid, and within the first 48 hours in 6 patients (17%) and 11 patients (29%), respectively.
LARYNGEAL ATTACKS
A total of 8 patients in FAST-1 and 3 patients in FAST-2 had laryngeal symptoms and received open-label icatibant; intubation was required in 1 of the FAST-2 patients 5 minutes after the icatibant was administered. Among these patients, the median time to first improvement as reported by the patient was 0.6 and 1.0 hours, respectively. As reported by an investigator, 7 of the 8 patients in FAST-1 and 2 of the 3 patients in FAST-2 had no symptoms at 4 hours after icatibant administration; the remaining patient in each study had mild symptoms at 4 hours. A total of 3 of the 8 FAST-1 patients with laryngeal symptoms received rescue medication, within 24 hours after icatibant administration, for new or recurrent symptoms. None of the 3 FAST-2 patients with laryngeal symptoms received rescue medication.
ADVERSE EVENTS
Major categories of adverse events occurring in the two trials are shown in Table 3; individual events classified by organ system are shown in Table 4 in the Supplementary Appendix. The most common adverse events were recurrent or worsening angioedema. Injection-site reactions, which were recorded separately from the other adverse events, were reported by the majority of patients in each study.
Table 3.
Adverse Events in the Safety Populations of the FAST-1 and FAST-2 Trials, According to Study Group.
| Adverse Event | FAST-1 | FAST-2 | ||
|---|---|---|---|---|
| Icatibant (N = 27) |
Placebo (N = 29) |
Icatibant (N = 36) |
Tranexamic Acid (N = 38) |
|
| no. of patients (%) | ||||
| Any adverse event | 12 (44) | 19 (66) | 19 (53) | 16 (42) |
| Drug-related adverse event* | 4 (15) | 1 (3) | 5 (14) | 4 (11) |
| Serious adverse event† | 0 | 0 | 4 (11) | 1 (3) |
| Injection-site reaction | 26 (96) | 8 (28) | 35 (97) | 10 (26) |
Drug-related adverse events in the icatibant groups were as follows: in the FAST-1 trial, injection-site pain in one patient, abnormal results on a liver-function test in one patient, dizziness in one patient, and nasal congestion in one patient; and in the FAST-2 trial, abdominal pain, nausea, and worsening of hereditary angioedema in one patient; asthenia in one patient; worsening of an acute attack of angioedema in one patient; injection-site reaction and two episodes of rash in one patient; and injection-site reaction in one patient.
Serious adverse events in the icatibant group in the FAST-2 trial were as follows: gastroenteritis and hypertensive crisis in one patient, laryngeal attack of angioedema in one patient, abdominal attack of angioedema in one patient, cholelithiasis in one patient, and laryngeal attack of angioedema requiring intubation in one patient. None were considered to be related to icatibant.
In the FAST-1 trial, 5 patients (9%) had a total of 5 adverse events considered to be related to the study drug. Of these, four events reported by 4 patients (15%) in the icatibant group were injection-site pain, abnormal results on a liver-function test (see the Supplementary Appendix), dizziness, and nasal congestion; 1 patient (3%) in the placebo group reported one event (injection-site irritation). In the FAST-2 trial, 5 patients (14%) receiving icatibant and 4 patients (11%) receiving tranexamic acid had a total of nine and six drug-related adverse events, respectively. For the 5 patients in the icatibant group, the events were abdominal pain, nausea, and worsening of an acute attack of angioedema in 1 patient; asthenia in 1 patient; injection-site reactions in 2 patients; and two episodes of rash in 1 patient.
No serious adverse events were reported during the controlled phase of the FAST-1 study. In the FAST-2 study, 5 patients (7%) had a total of 8 serious adverse events (see the Supplementary Appendix); none were considered to be related to the study drug. No patient discontinued either study because of an adverse event.
DISCUSSION
We evaluated the effect of icatibant, a bradykinin B2 receptor antagonist, in patients with hereditary angioedema in two randomized, controlled clinical trials. In both trials, icatibant or a comparison study drug was administered at the time of presentation with an acute attack of angioedema. In the FAST-1 trial, the primary end point, or the time to clinically significant relief of the index symptom, was 2.5 hours for patients receiving icatibant and 4.6 hours for patients receiving placebo, a difference that was not significant. In the FAST-2 trial, the time to clinically significant relief of the index symptom was 2.0 hours for the patients receiving icatibant and 12.0 hours for the patients receiving tranexamic acid, a difference that was significant. The time to first improvement, as assessed subjectively, by both the patient and the investigator, was significantly faster with icatibant in both trials, although the results of two other prespecified secondary end points differed between the two trials.
We believe that the rate of the primary end point did not differ significantly between the two groups in FAST-1 because of the stringent definition of that end point and its analysis — in particular, the specification that only the index symptom (and not other symptoms) be assessed in defining symptom relief and the inclusion of data from patients in the placebo group who received rescue medication early. In a post hoc analysis that censored data from patients who received rescue medication, a significant benefit of icatibant as compared with placebo was shown (Table 2 in the Supplementary Appendix). This was also true for an exploratory analysis of all three index symptoms together, even when the end point of time to symptom relief was conservatively defined as a reduction of the baseline score on the visual-analogue scale of more than 50% (Fig. 4 in the Supplementary Appendix). On the basis of the published literature and clinical experience, it is very unlikely that the difference in results regarding the primary end point between FAST-1 and FAST-2 is due to a harmful effect of tranexamic acid.
A further possible explanation is that the FAST-1 trial was underpowered to detect a clinically relevant difference in the primary end point. As noted, the trial was powered on the basis of the assumption that the median difference in the time to clinically significant relief of the index symptom would be 5.5 hours; the actual difference in median values was 2.1 hours. This difference, if real, would be clinically important.
No serious drug-related adverse events were reported in either trial, and no patient discontinued either study owing to an adverse event. Although most patients receiving icatibant had an injection-site reaction, these were generally mild or moderate in severity and short-lived and resolved spontaneously. Despite the theoretical concern that icatibant might alter blood pressure owing to antagonism of the vasodilating properties of brady-kinin,19 the use of icatibant did not increase blood pressure and had no effect on heart rate. The fact that recurrent or worsening angioedema was the most commonly reported adverse event suggests that, in clinical practice, additional medication (either another dose of icatibant or another agent) would likely be necessary in some patients to achieve complete control of symptoms.
In summary, we evaluated the effect of icatibant for the treatment of acute attacks of angioedema in patients with hereditary angioedema. In the FAST-1 trial, there was a nonsignificant difference in the primary end point for patients given icatibant and patients given placebo. In the FAST-2 trial, the time to clinically significant relief of the index symptom was significantly shorter for patients given icatibant than for patients given tranexamic acid. The early use of rescue medication may have obscured a benefit of icatibant in the FAST-1 trial. To clarify the effect of icatibant as compared with placebo in the treatment of acute attacks of angioedema, a larger trial will be necessary.
Supplementary Material
Acknowledgments
Supported by Jerini and, for the FAST-1 trial only, in part by a grant from the National Institutes of Health (M01 RR-00051).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the staff and coinvestigators at participating centers and all the collaborators of the sponsor, Jerini. Assistance with medical writing during the preparation of this manuscript was provided by Carl V. Felton, Ph.D., at Prime Healthcare, who was supported by Jerini.
APPENDIX
The authors’ full names and degrees are as follows: Marco Cicardi, M.D., Aleena Banerji, M.D., Francisco Bracho, M.D., Alejandro Malbrán, M.D., Bernd Rosenkranz, M.D., Marc Riedl, M.D., Konrad Bork, M.D., William Lumry, M.D., Werner Aberer, M.D., Henning Bier, M.D., Murat Bas, M.D., Jens Greve, M.D., Thomas K. Hoffmann, M.D., Henriette Farkas, M.D., Avner Reshef, M.D., Bruce Ritchie, M.D., William Yang, M.D., Jürgen Grabbe, M.D., Shmuel Kivity, M.D., Wolfhart Kreuz, M.D., Robyn J. Levy, M.D., Thomas Luger, M.D., Krystyna Obtulowicz, M.D., Peter Schmid-Grendelmeier, M.D., Christian Bull, M.D., Brigita Sitkauskiene, M.D., William B. Smith, M.B., B.S., Ph.D., Elias Toubi, M.D., Sonja Werner, M.D., Suresh Anné, M.D., Janne Björkander, M.D., Laurence Bouillet, M.D., Enrico Cillari, M.D., David Hurewitz, M.D., Kraig W. Jacobson, M.D., Constance H. Katelaris, M.D., Marcus Maurer, M.D., Hans Merk, M.D., Jonathan A. Bernstein, M.D., Conleth Feighery, M.D., Bernard Floccard, M.D., Gerald Gleich, M.D., Jacques Hébert, M.D., Martin Kaatz, M.D., Paul Keith, M.D., Charles H. Kirkpatrick, M.D., David Langton, M.D., Ludovic Martin, M.D., Christiane Pichler, M.D., David Resnick, M.D., Duane Wombolt, M.D., Diego S. Fernández Romero, M.D., Andrea Zanichelli, M.D., Francesco Arcoleo, M.D., Jochen Knolle, Ph.D., Irina Kravec, M.D., Liying Dong, M.D., Jens Zimmermann, M.D., Kimberly Rosen, M.D., and Wing-Tze Fan, Ph.D.
The authors’ affiliations are as follows: Università degli Studi di Milano, Milan (M.C., A.Z.); Massachusetts General Hospital and Harvard Medical School — both in Boston (A.B.); Georgetown University Hospital, Lombardi Cancer Center, Washington, DC (F.B.); Hospital Británico de Buenos Aires, Buenos Aires (A.M., D.S.F.R.); Jerini, Berlin (B. Rosenkranz, J.K., I.K., L.D., J.Z., K.R., W.-T.F.), University of Mainz, Mainz (K.B.), Technische Universität München, Munich (H.B., M.B.), University of Düsseldorf, Düsseldorf (J. Greve, T.K.H.), University Hospital Lübeck, Lübeck (J. Grabbe), Wolfgang Goethe University, Frankfurt (W.K.), University Hospital, Munster (T.L.), Charité Universitätsmedizin, Berlin (M.M.), University Hospital Rheinisch Westfälische Technische Hochschule Aachen, Aachen (H.M.), and Friedrich Schiller Universität Jena, Jena (M.K.) — all in Germany; UCLA David Geffen School of Medicine, Los Angeles (M.R.); Asthma, Allergy Research Associates Research Center, Dallas (W.L.); Medical University of Graz, Graz, Austria (W.A.); Semmelweis University, Budapest, Hungary (H.F.); Sheba Medical Center, Tel-Hashomer (A.R.), Tel Aviv Sourasky Medical Center, Tel Aviv (S.K.), and Bnai-Zion Medical Center, Haifa (E.T.) — all in Israel; University of Alberta, Edmonton (B. Ritchie), Allergy and Asthma Research Centre, Ottawa (W.Y.), Centre de Recherche Appliquée en Allergie de Québec, Quebec City, QC (J.H.), and McMaster University, Hamilton Health Sciences Center, Hamilton, ON (P.K.) — all in Canada; Family Allergy and Asthma Center, Atlanta (R.J.L.); Szpital Uniwersytecki w Krakowie, Krakow, Poland (K.O.); University Hospital Zürich, Zurich (P.S.-G., C.B.), and Insel Spital BE Bern (C.P.) — both in Switzerland; Kaunas University of Medicine, Kaunas, Lithuania (B.S.); Royal Adelaide Hospital, Clinical Immunology and Allergy, Adelaide, SA (W.B.S.), Westmead Hospital, Sydney (C.H. Katelaris), and Frankston Hospital, Frankston, VIC (D.L.) — all in Australia; Universitetssjukhuset i Lund, Lund (S.W.), and Sahlgrenska Universitetssjukhuset, Gothenborg (J.B.) — both in Sweden; Asthma, Allergy, and Immunology Center, Flint, MI (S.A.); Centre Hospitalier Universitaire de Grenoble, Grenoble (L.B.), Hôpital Edouard Herriot, Lyon (B.F.), and Centre Hospitalier Regional d’Orléans, Orleans (L.M.) — all in France; Azienda Ospedaliera V. Cervello, Palermo, Italy (E.C., F.A.); Allergy Clinic of Tulsa, Tulsa, OK (D.H.); Allergy and Asthma Research Group, Eugene, OR (K.W.J.); University of Cincinnati, Cincinnati (J.A.B.); St. James’s Hospital, Dublin (C.F.); University of Utah Health Sciences Center, Salt Lake City (G.G.); University of Colorado Health Science Center, Denver (C.H. Kirkpatrick); Columbia University Medical Center, New York (D.R.); and Clinical Research Associates of Tidewater, Norfolk, VA (D.W.).
REFERENCES
- 1.Bork K, Frank J, Grundt B, Schlattmann P, Nussberger J, Kreuz W. Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant) J Allergy Clin Immunol. 2007;119:1497–1503. doi: 10.1016/j.jaci.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angiooedema. Lancet. 1998;351:1693–1697. doi: 10.1016/S0140-6736(97)09137-X. [DOI] [PubMed] [Google Scholar]
- 3.Cugno M, Nussberger J, Cicardi M, Agostoni A. Bradykinin and the pathophysiology of angioedema. Int Immunopharmacol. 2003;3:311–317. doi: 10.1016/S1567-5769(02)00162-5. [DOI] [PubMed] [Google Scholar]
- 4.Han ED, MacFarlane RC, Mulligan AN, Scafidi J, Davis AE., III Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J Clin Invest. 2002;109:1057–1063. doi: 10.1172/JCI14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med. 1996;334:1666–1667. doi: 10.1056/NEJM199606203342510. [DOI] [PubMed] [Google Scholar]
- 6.Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161:2417–2429. doi: 10.1001/archinte.161.20.2417. [DOI] [PubMed] [Google Scholar]
- 7.Sachse MM, Khachemoune A, Guld-bakke KK, Kirschfink M. Hereditary angioedema. J Drugs Dermatol. 2006;5:848–852. [PubMed] [Google Scholar]
- 8.Bork K, Siedlecki K, Bosch S, Schopf RE, Kreuz W. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc. 2000;75:349–354. doi: 10.4065/75.4.349. [DOI] [PubMed] [Google Scholar]
- 9.Ohela K. Treatment of hereditary angioneurotic edema with tranexamic acid and cinnarizine. Acta Derm Venereol. 1976;56:61–67. [PubMed] [Google Scholar]
- 10.Sheffer AL, Austen KF, Rosen FS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med. 1972;287:452–454. doi: 10.1056/NEJM197208312870907. [DOI] [PubMed] [Google Scholar]
- 11.Zuraw BL. Hereditary angioedema. N Engl J Med. 2008;359:1027–1036. doi: 10.1056/NEJMcp0803977. [DOI] [PubMed] [Google Scholar]
- 12.Cugno M, Zanichelli A, Foieni F, Cac-cia S, Cicardi M. C1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progress. Trends Mol Med. 2009;15:69–78. doi: 10.1016/j.molmed.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Waytes AT, Rosen FS, Frank MM. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med. 1996;334:1630–1634. doi: 10.1056/NEJM199606203342503. [DOI] [PubMed] [Google Scholar]
- 14.Bork K, Barnstedt SE. Treatment of 193 episodes of laryngeal edema with C1 inhibitor concentrate in patients with hereditary angioedema. Arch Intern Med. 2001;161:714–718. doi: 10.1001/archinte.161.5.714. [DOI] [PubMed] [Google Scholar]
- 15.Wirth KJ, Heitsch H, Schölkens BA. Kinin receptor antagonists: unique probes in basic and clinical research. Can J Physiol Pharmacol. 1995;73:797–804. doi: 10.1139/y95-108. [DOI] [PubMed] [Google Scholar]
- 16.Fincham CI, Bressan A, Paris M, Rossi C, Fattori D. Bradykinin receptor antagonists — a review of the patent literature 2005–2008. Expert Opin Ther Pat. 2009;19:919–941. doi: 10.1517/13543770902994389. [DOI] [PubMed] [Google Scholar]
- 17.Rhaleb NE, Rouissi N, Jukic D, et al. Pharmacological characterization of a new highly potent B2 receptor antagonist (HOE 140: D-Arg-[Hyp3,Thi5,D-Tic7,Qic8] bradykinin) Eur J Pharmacol. 1992;210:115–120. doi: 10.1016/0014-2999(92)90661-m. [DOI] [PubMed] [Google Scholar]
- 18.Davis AE., III Mechanism of angioedema in first complement component inhibitor deficiency. Immunol Allergy Clin North Am. 2006;26:633–651. doi: 10.1016/j.iac.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Cockcroft JR, Chowienczyk PJ, Brett SE, Bender N, Ritter JM. Inhibition of bradykinin-induced vasodilation in human forearm vasculature by icatibant, a potent B2-receptor antagonist. Br J Clin Pharmacol. 1994;38:317–321. doi: 10.1111/j.1365-2125.1994.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenkranz B, Brunner-Ferber F, Knolle J. Efficacy and safety profile of the potent and selective bradykinin B2 receptor antagonist icatibant in healthy volunteers; Presented at the 26th EAACI Congress; June 9–13, 2007; Gothenburg, Sweden. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.