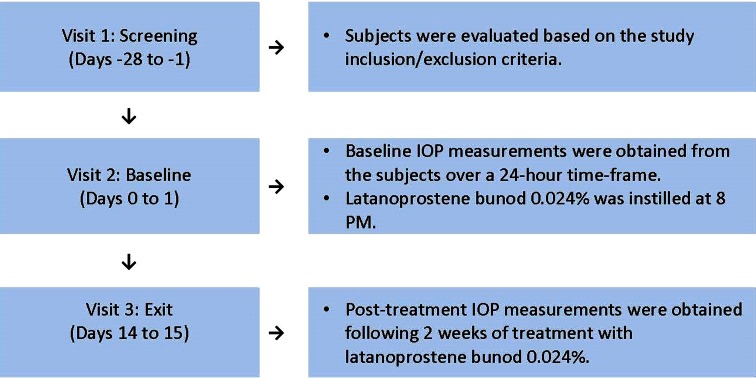
Fig. 1.
Schematic of the clinical study design. Three study visits were required for all subjects to complete the study. Measurements of intraocular pressure (IOP) were recorded from both eyes for each subject at Visit 2 and Visit 3 at nine time points (8 PM, 10 PM, 12 AM, 2 AM, 4 AM, 8 AM, 10 AM, 12 PM, and 4 PM). Latanoprostene bunod 0.024% was provided to all subjects following the 4 PM IOP assessments at Visit 2, with instructions to instill the study drug QD at 8 PM