Abstract
Background and Objectives:
The main cause of serious nosocomial infections is a Gram-negative pathogen known as Pseudomonas aeruginosa (P. aeruginosa). Carbapenems are widely used as an appropriate treatment for these infections, however resistance to these agents has been observed and is increasing. Metallo beta-lactamase (MBLs) enzyme is one of the main causes of resistance to carbapenem. In the current study the frequency and production of VIM1 and VIM2 by imipenem-resistant P. aeruginosa isolates of patients hospitalized in Imam Reza hospital were evaluated.
Materials and Methods:
In this study, 131 clinical samples were collected from patients hospitalized in Imam Reza hospital in Mashhad during a 15-month period from May 2011 to November 2012. After verification of P. aeruginosa isolates, antibiotic resistance patterns of isolates were determined for 14 antibiotics by Kirby-Bauer standard disk diffusion according to the CLSI guidelines. Combined-disk test was used for phenotypic determination of MBLs-producing isolates and after DNA extraction, genotypic determination of VIM1 and VIM2 metallo beta-lactamase genes was carried out using Multiplex-PCR.
Results:
Of 63 imipenem-resistant isolates (48.5%), 56 (88.8%) were MBL-producing in phenotypic assessments. Also amongst imipenem-resistant isolates, the frequency of VIM1 and VIM2 genes were 58.7 and 3.17%, respectively.
Conclusion:
The results of the current study along with the results of the other conducted studies in Iran in recent years demonstrate that the average resistance to imipenem in P. aeruginosa isolates was 51.3% which has increased in comparison with the results in 2006 (32.9%). It was also determined that the frequency of VIM1 gene was more than VIM2 gene. In phenotypic assessment by using CD method, 49.6% of isolates were determined as MBLs-producing. The sensitivity and specificity of this method were verified in comparison with the results of PCR test.
Keywords: Pseudomonas aeruginosa, Imipenem, Metallobeta-lactamase genes
INTRODUCTION
Pseudomonas aeruginosa (P. aeruginosa) is responsible for a wide spectrum of nosocomial infections including pneumonia, urinary infections, postoperative bacteremia, and wound infection. According to several reports published from 1997 to 2003, the prevalence of P. aeruginosa-induced nosocomial infections has been increased. P. aeruginosa is the second most prevalent pathogen isolated from patients hospitalized in ICU, after Staphylococcus aureus (1). Due to the low permeability of the outer membrane of P. aeruginosa, it is sensitive to a limited number of antimicrobials. As a result, the infections caused by them could not be easily treated. Resistant strains of P. aeruginosa are a great threat to the health of patients (2).
Amongst betalactam anitibiotics which are suitable for the treatment of those infections caused by Gram-negative bacteria carbapenems including imipenem and meropenem are considered as effective antibiotics for the treatment of these infections due to its’ broad-spectrum effect and resistance to beta-lactamase enzymes. However, resistance to these antibiotics is increasing (3).
Various mechanisms contribute to drug resistance in P. aeruginosa (2). The main mechanism responsible for resistance to imipenem in P. aeruginosa, is losing porin D gene in outer membrane of bacterium due to mutations. Mutations in porin D gene following the treatment with imipenem is prevalent and a 25% increase in imipenem resistance has been observed in patients (4, 5).
Another reason for resistance to carbapenem is the production of metallo beta-lactamase enzymes which can be either chromosomal or plasmid mediated. The most prevalent types of these enzymes are IMP, VIM, GIM, and SPM. Amongst metallo beta-lactamase, IMP and VIM are prevalent worldwide. Also MBL genes are located on plasmids which can be transferred to other Gram-negative bacteria (6–8). Therefore, using phenotypic and genotypic methods, which rapidly detect metallo beta-lactamase-producing bacteria, can help physicians to begin the most suitable treatment as soon as possible and to prevent the transfer of resistance to other bacteria. Currently there is no available data regarding the prevalence of MBL-producing P. aeruginosa among patients hospitalized in Imam Reza hospital. Therefore, this study was conducted to determine the frequency of VIM1 and VIM2 metallo beta-lactamase produced by imipenem-resistant P. aeruginosa.
MATERIALS AND METHODS
In the current study, 131 clinical samples were collected from May 2011 to November 2012 from hospitalized patients at Imam Reza hospital in Mashhad, Iran. P. aeruginosa was isolated by using standard microbiological methods including Gram staining, culturing on MacConkey agar medium, oxidase tests, catalase tests, MRVP, SIM, TSI, OF, culturing at 42°C, and production of green-blue pigments. For further analysis, isolates were inoculated into glycerol-containing BHI culture and were stored at −70 °C (9).
Antibiotic resistance pattern of isolates were determined for 14 antibiotics (MAST-UK) using standard Kirby-Bauer Disk Diffusion method. Medium was prepared and sterilized by autoclaving at 121° for 15 min. 25 ml of media was poured in 90 mm sterile Petri dishes and incubated at 37°C overnight in order to check sterility. Antibiotics used in the current study were as following: ceftriaxone, ciprofloxacin, gentamycin, cefixime, meropenem, cephtazidiom, tetracycline, imipenem, colistin, amikacin, cefepime, carbenicillin, piperacilline, and Tobramycin. Then, the plates were incubated at 37°C for 24h and finally inhibition zone surrounding the disk were measured and sensitive, intermediate, resistant isolates were assigned according to the CLSI guideline (10).
Phenotypic detection of MBL-producing isolates by using Combined-Disk (CD) method.
In this study, combined-disk test was used in order to phenotypically distinguish MBL-producing isolates. First, 18.61 gr of disodium EDTA. 2H2O was dissolved in 100 ml of distilled water. Then its pH was adjusted to 8 by adding Sodium Hydroxide and was then autoclaved to prepare 0.5 M EDTA (Sigma, USA). Microbial suspension with the turbidity of equal to 0.5 McFarland was prepared on Brain Heart Infusion (BHI) medium and then the suspension was cultured on Muller Hinton agar medium after incubation for 20 minutes. After that, an imipenem disk and an imipenem disk containing 10μl of 0.5 M EDTA was located on the plate with 2 cm distance from each other and were incubated for 16–18 h at 35 °C. More than 7mm increase in the diameter of inhibition zone around the EDTA-containing imipenem disk in comparison with imipenem disk demonstrated the production of MBL by bacterium (11, 12).
P. aeruginosa ATCC27853 was used as a control during antibiogram and production of MBL tests.
Determining MBL gene by using Multiplex PCR.
DNA of bacterial isolates were extracted by simple boiling method in order to perform genotypic tests (13). VIM1 and VIM2 primers were designed in accordance with nucleotide sequence mentioned in Table 1. Optimized PCR was performed in order to determine blaVIM1 (261 bp) and blaVIM2 (798 bp) genes. The mixture of the main solution with the volume of 20 μl was as following: 10X PCR buffer, 0.4 μl dNTP, 1.25 μl Magnesium Chloride (50 Mm), 0.2 μl Taq DNA polymerase, 0.8 μl of each 10 pM primers, 1 μl of DNA template, and 13.55 μl distilled water. PCR program was performed for 35 cycles which was as following: The first step was denaturation for 5 min at 94 °C, then 35 cycles including 30 sec at 94 °C, 40 sec at 55 °C, and 50 seconds at 72 °C, and final elongation step for 5 min at 72 °C.
Table 1.
Nucleotide sequences of primers blaVIM1 and blaVIM2
| Primers | (Nucleotide) sequence |
|---|---|
| VIM1-F | 5′-AGTGGTGAGTATCCGACAG-3′ |
| VIM1-R | 5′-ATGAAAGTGCGTGGAGAC-3′ |
| VIM2-F | 5′-ATGTTCAAACTTTTGAGTAAG-3′ |
| VIM2-R | 5′-CTCAACGACTGAGCGATTG-3′ |
PCR products were assessed by performing electrophoresis on 1.5% agarose gel in TBE buffer. Finally, gels were stained with Ethidium Bromide and UV light was used to visualize PCR products.
RESULTS
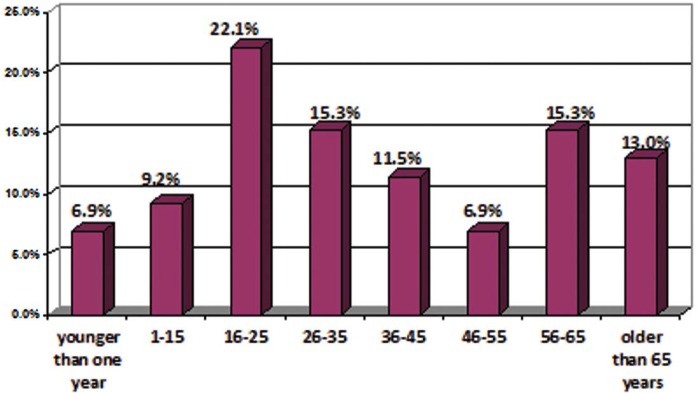
Of 131 patients hospitalized from whom P. aeruginosa isolates were cultured in Imam Reza hospital, 46.6% (n= 61) and 53.4% (n= 70) were male and female, respectively. Age distribution of patients is given in diagram (Fig. 1). Also the frequency of patients in different wards are listed in Table 2. The majority of patients were hospitalized in the burn ward.
Fig. 1.
Age distribution of 131 hospitalized patients of Imam Reza hospital, Mashhad, Iran
Table 2.
Prevalence of distribution of samples from patients of different wards of Imam Reza hospital, Mashhad, Iran
| Sample | Total | ||||||
|---|---|---|---|---|---|---|---|
| Urine | Bronchi | Wound | Other | Blood culture | |||
| ICU | Number | 1 | 12 | 4 | 8 | 1 | 26 |
| Percentage | 3.8% | 46.2% | 15.4% | 30.8% | 3.8% | 100.0% | |
| Other Ward | Number | 3 | 2 | 3 | 7 | 9 | 24 |
| Percentage | 12.5% | 8.3% | 12.5% | 29.2% | 37.5% | 100.0% | |
| Iutera Disease | Number | 3 | 1 | 5 | 7 | 4 | 20 |
| Percentage | 15.0% | 5.0% | 25.0% | 35.0% | 20.0% | 100.0% | |
| Burn | Number | 1 | 0 | 57 | 0 | 3 | 61 |
| Percentage | 1.6% | 0.0% | 93.4% | 0.0% | 4.9% | 100.0% | |
| Total | Number | 8 | 15 | 69 | 22 | 17 | 131 |
| Percentage | 6.1% | 11.5% | 52.7% | 16.8% | 13.0% | 100.0% | |
Results of drug susceptibility testing of isolates is demonstrated in Table 3.
Table 3.
Results of susceptibility testing on isolates cultured from patients with nosocomial infection at Imam Reza Hospital
| Antibiotic | Sensitive | Semi-Sensitive | Resistance | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | Number | % | |
| Amikacin | 55 | 51.4% | 16 | 15.0% | 36 | 33.6% | 107 | 100.0% |
| Ceftriaxon | 14 | 23.7% | 7 | 11.9% | 38 | 64.4% | 59 | 100.0% |
| Cephtazideme | 22 | 22.2% | 15 | 15.2% | 62 | 62.6% | 99 | 100.0% |
| Cefepime | 16 | 13.4% | 11 | 9.2% | 92 | 77.3% | 119 | 100.0% |
| Cefixime | 3 | 4.4% | 4 | 5.9% | 61 | 89.7% | 68 | 100.0% |
| Ciprofloxacin | 79 | 63.2% | 7 | 5.6% | 39 | 31.2% | 125 | 100.0% |
| Gentamicin | 47 | 37.0% | 11 | 8.7% | 69 | 54.3% | 127 | 100.0% |
| Meropenem | 7 | 8.1% | 3 | 3.5% | 76 | 88.4% | 86 | 100.0% |
| Piperacillin | 55 | 47.8% | 13 | 11.3% | 47 | 40.9% | 115 | 100.0% |
| Tetracyclin | 9 | 22.5% | 5 | 12.5% | 26 | 60.0% | 40 | 100.0% |
| Imipenem | 61 | 46.9% | 6 | 4.6% | 63 | 48.5% | 130 | 100.0% |
| Carbenicillin | 1 | 25.0% | 0 | .0% | 3 | 75.0% | 4 | 100.0% |
| Tobramycin | 0 | .0% | 0 | .0% | 2 | 100.0% | 2 | 100.0% |
| Colistin | 61 | 78.2% | 17 | 21.8% | 0 | .0% | 78 | 100.0% |
In phenotypic assessment of MBL production, 49.6 % (n= 65) of isolates were MBL-producing. In genotypic assessment performed by PCR, 55% (n= 72) of isolates were determined as MBL-producing isolates and frequency distribution of VIM1 and VIM2 genes are shown in Table 4.
Table 4.
Distribution of metallo-beta-lactamase genes VIM1 and VIM2 among isolated P. aeruginosa samples of hospitalized patients of Imam Reza hospital in Mashhad, Iran
| VIM | Number | Percentage |
|---|---|---|
| VIM1(−) ---- VIM2(−) | 59 | 45.0% |
| VIM1(−) ---- VIM2(+) | 2 | 1.5% |
| VIM1(+) ---- VIM2(−) | 64 | 48.9% |
| VIM1(+) ---- VIM2(+) | 6 | 4.6% |
| Total | 131 | 100.0% |
Increase in diameter of inhibitory zone (≥7mm) in EDTA-containing imipenem disks demonstrates the production of MBL by the bacterium. Of 63 (48.5%) imipenem-resistant strains, 56 (88.8%) strains were MBL-producing in phenotypic assessments. Furthermore, amongst imipenem-resistant strains, the presence of VIM1 and VIM 2 were 58.7 and 3.17%, respectively.
DISCUSSION
Infection with Gram-negative bacteria is one of the main causes of nosocomial infections. P. aeruginosa is the most noticeable causes of these infections which contribute to high rates of mortality rate due to its resistance to the broad spectrum antibiotics. Since metallo beta-lactamase (MBL) is one of the most important beta lactamase-hydrolyzing enzymes and could be easily transferred to other bacteria, using appropriate methods to rapid determination of MBL-producing bacteria will help physicians to choose the most appropriate treatment and hence to prevent the transmission of resistance to other bacteria (14).
In the current study, 48.5% of isolates were resistant to imipenem with 88.8% being MBL-producing . In comparison of these results with the study of Khosravi et al., the rate of resistance to imipenem was relatively similar (48.5% in the city of Mashhad and 41% in the city of Ahwaz in Iran) but MBL-producing strains in Mashhad was significantly higher than that of Ahvaz (in the city of Mashhad: 88.8%, and in the city of Ahwaz in Iran: 19.5%) indicating that the production of MBL is one of the main mechanisms for resistance to imipenem in P. aeruginosa strains isolated from patients in Mashhad (15).
In a study by Saderi et al., in Tehran, among 100 isolates of P. aeruginosa, 69 (69%) were resistant to imipenem, of which, 65 (94.2%) had metallo beta lactamase activity (determined by phenotypic detection of metallo beta lactamase production). Using PCR, 13 (18.84%) of isolates had VIM2 gene and none of them had VIM1 gene (11). Compared to our results, a similar frequency of imipenem resistance was observed for P. aeruginosa. However, metallo beta lactamase production in their study was lower than that of the current study. In the present study, VIM1 gene was observed in 58.7% of isolates, however, that of Sadri et al ., did not find the presence of this gene in any of the isolates. In a study by Euh-jee Oh in South Korea in 2003 on 99 isolates of P. aeruginosa, CD phenotypic test was shown to have a high specificity and sensitivity for detection of metallo beta lactamase production. Using PCR, VIM2 gene was detected in 29 (29.3%) of isolates. This study showed a higher frequency of VIM2 than that of ours (16).
The results of the current study along with results of other conducted studies in Iran suggest that the mean resistance to imipenem in P. aeruginosa strains isolated from patients is 51.3% which has increased in comparison with 2006 which was reported to be 32.9% (9, 13, 15, 17).
Ting-ting Qu et al. performed a study in 2009 to evaluate phonotypic methods of P. aeruginosa isolates capable of producing metallobeta Lactamase. Three phenotypic methods of Etest, DDST and CD were used to detect metallobeta lactamase production among 264 isolates of imipenem-resistant strains. Of which, 24 (9.1%) showed positive results. This was confirmed by PCR and DNA sequencing procedures. The study showed that CD was the most suitable phenotypic test for detection of P. aeruginosa isolates capable of producing metallobeta lactamase (18).
In phenotypic assessment with CD method, 49.6% of samples were MBL-producing. The sensitivity and specificity of this method were verified in comparison with the results of PCR method in Table 1.
Results of current study suggests increase in MBL-producing P. aeruginosa isolates and also increase in drug resistance among these strains. Therefore, prescribing appropriate antibiotics and also quick determination of MBL-producing strains are vital in order to prevent the transfer of drug resistance to other bacteria. According to comparison of CD phenotypic test and PCR in evaluation of MBL-producing strains, CD phenotypic test could be used as a simple and cost-effective method in laboratory experiments. Molecular typing of these strains based on MBL genes is one of the aims of this similar projects in future.
ACKNOWLEDGMENTS
The current study was financially supported by the Deputy of Research, Mashhad University of Medical Sciences. The authors are thankful to staff at Imam Reza hospital and Microbiology laboratory of Buali Research Institute.
REFERENCES
- 1. Streit JM, Jones RN, Sader HS, Fritsche TR. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001). Int J Antimicrob Agents 2004; 24: 111– 118. [DOI] [PubMed] [Google Scholar]
- 2. PA L. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. JRSM 2002; 25: 22. [PMC free article] [PubMed] [Google Scholar]
- 3. Bush K JG, Medeiros AA. A functional classification scheme for b-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother 1995;39: 1211– 1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cometta A BJ, Lew D, Zimmerli W, Pittet D, Chopart P, Schaad U, Herter C, Eggimann P, Huber O. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother 1994; 38: 1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zanetti G, Bally F, Greub G, Garbino J, Kinge T, Lew D, et al. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob Agents Chemother 2003; 47: 3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strateva T, Yordanov D. Pseudomonas aeruginosa–a phenomenon of bacterial resistance. J Med Microbiol 2009;58: 1133– 1148. [DOI] [PubMed] [Google Scholar]
- 7. Nordmann P, Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clin Microbiol Infect 2002;8: 321– 331. [DOI] [PubMed] [Google Scholar]
- 8. Toleman MA, Simm AM, Murphy TA, Gales AC, Biedenbach DJ, Jones RN, et al. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial surveillance programme. J Antimicrob Chemother 2002;50: 673– 679. [DOI] [PubMed] [Google Scholar]
- 9. Jamali SH, Bahar MA, Hooshmand SM. Prevalence of bla VIM and bla IMP metallo-β-lactamase genes in imipenem-resistant Pseudomonas aeruginosa strains isolated from burn patients in Motahari Tehran hospital. Daneshe Microbshenasi 2008; 1: 1–7 [In persian]. [Google Scholar]
- 10. Clinical and Laboratory standards institute (CLSI) Performance standards for antimicrobial susceptibility testing, 16th informational supplements. CLSI Document M2-A9, Wayne PA: 2006. [Google Scholar]
- 11. Saderi H, Lotfalipour H, Owlia P, Salimi H. Detection of Metallo-β-Lactamase Producing Pseudomonas aeruginosa Isolated From Burn Patients in Tehran, Iran. Lab Medicine 2010;41: 609– 12. [Google Scholar]
- 12. Franklin C, Liolios L, Peleg AY. Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing gram-negative bacilli in the clinical laboratory. J Clin Microbiol 2006; 44: 3139– 3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sepehri Seresht S, Najar Peerayeh S, Sattari M, Rezaee MA. Production of plasmid-mediated ß-lactamases in Pseudomonas aeruginosa isolated from burnpatients. Hakim Research Journal 2007;10: 61– 65. [Google Scholar]
- 14. Sacha P, Wieczorek P, Hauschild T, Żórawski M, Olszańska D, Tryniszewska E. Metallo-β-lactamases of Pseudomonas aeruginosa-a novel mechanism resistance to β-lactam antibiotics. Folia Histochem Cytobiol 2008;46: 137– 142. [DOI] [PubMed] [Google Scholar]
- 15. Khosravi AD, Mihani F. detection of metallo-b-lactamase producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis 2008;60: 125– 128. [DOI] [PubMed] [Google Scholar]
- 16. Oh E-J, Lee S, Park Y-J, Park JJ, Park K, Kim S-I, et al. Prevalence of metallo-β-lactamase among Pseudomonas aeruginosa and Acinetobacter baumannii in a Korean University hospital and comparison of screening methods for detecting metallo-β-lactamase. J Microbiol Methods. 2003;54: 411– 418. [DOI] [PubMed] [Google Scholar]
- 17. Mohajeri P. Antibiotic susceptibility and resistance patterns of pseudomonas aeruginosa strains isolated from different clinical specimens in patients referred to the teaching hospitals in Kermanshah (2001–2). Behbood Res J Kermanshah Univ Med Sci 2004; 7(4). [Google Scholar]
- 18. Qu T-t, Zhang J-l, Wang J, Tao J, Yu Y-s, Chen Y-g, et al. Evaluation of phenotypic tests for detection of Metallo-β-lactamase-producing Pseudomonas aeruginosa strains in China. J Clin Microbiol 2009;47: 1136– 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]