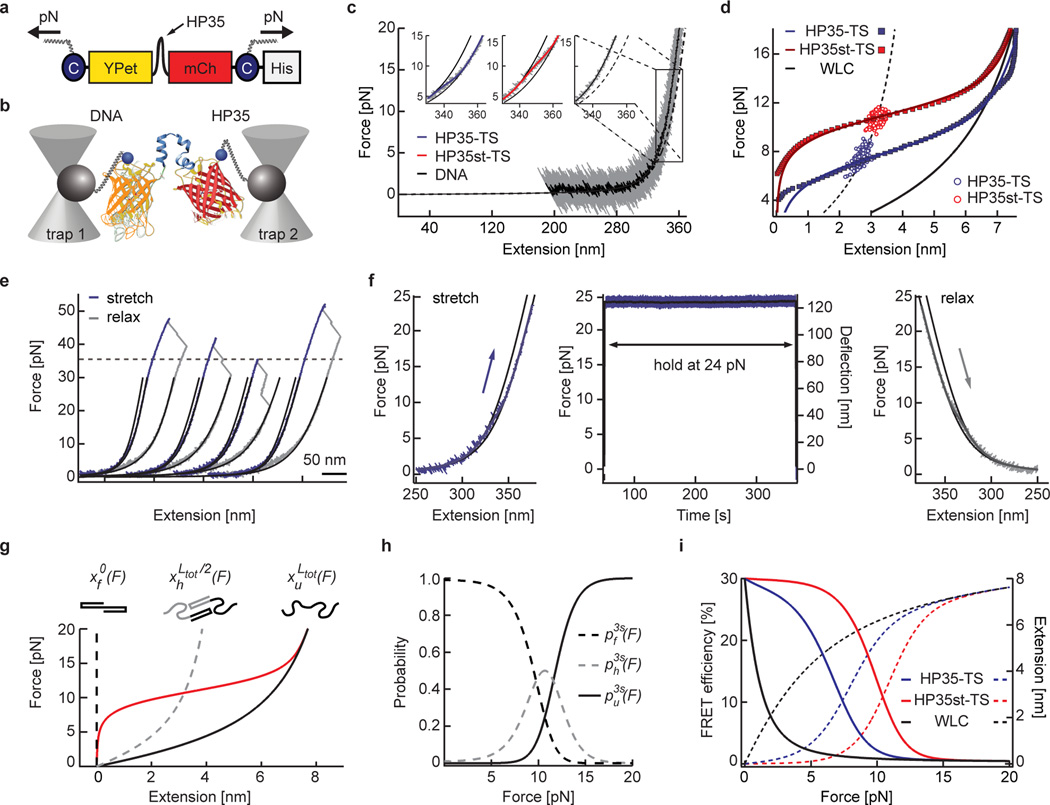
Figure 1.
Biosensor calibration using single-molecule force spectroscopy. (a) HP35-TS comprises two fluorophores, YPet and mCherry (mCh), which are linked by the villin headpiece peptide (HP35). Mechanical force across this biosensor leads to HP35 unfolding, increase in fluorophore separation distance and reduced FRET. For single-molecule (sm) calibration, DNA handles were attached using cysteines (C), a His-tag was used for purification. (b) Schematic illustration of the custom-built dual trap optical tweezer setup used for calibration. (c) 200 kHz resolution force-extension trace (FE) (gray) fitted with an extensible worm-like chain model (black). Inset: Zoom into representative FEs of individual HP35(st)-TS molecules as compared to DNA; the fit to HP35-TS data is shown in blue, HP35-st-TS in red and DNA in black. (d) Average FE of individual HP35-TS (blue) and HP35st-TS (red) molecules. Experimental data are shown as filled squares, solid lines are fits to the data, empty circles represent transition mid-point forces (HP35-TS: n=344 single pulls pooled from 15 independent repeats, i.e. different molecules; HP35st-TS: n=338 single pulls pooled from 10 independent repeats). (e) FE of four representative HP35-TS molecules showing fluorophore unfolding at high (>35 pN) forces. (f) After stretching to 24 pN, HP35-TS was exposed to high force for more than five min before relaxation; no indications of fluorophore unfolding were observed. (g) Average force-extension fit for HP35st-TS using a three-state model. The dashed black line represents the folded state, the gray dashed line the half-folded/half-unfolded state with contour length and the black solid line the completely unfolded state with contour length ; the red line indicates the average protein extension (h) Probability plot for the folded, half-folded/half-unfolded and unfolded state. (i) Modelled FRET-force (solid lines) and extension-force (dashed lines) correlations of HP35(st)-TS.