Figure 3. Hepatic Baf60a regulates bile acid metabolism and cholesterol absorption.
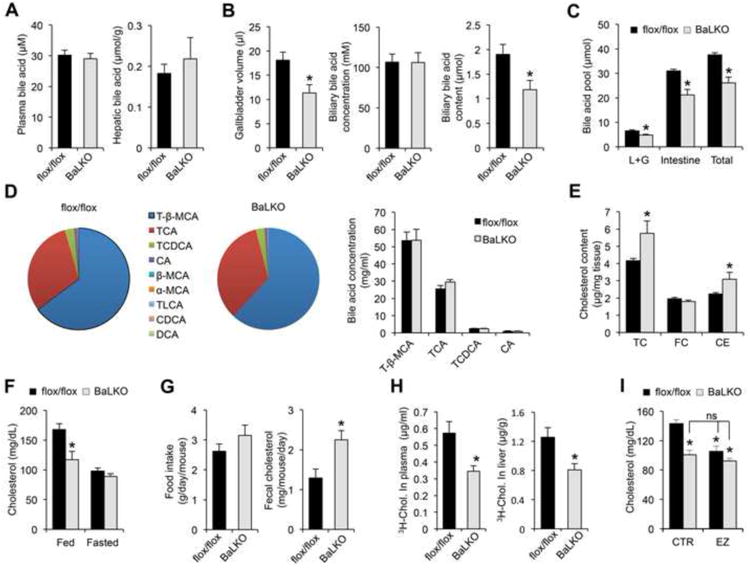
flox/flox and BaLKO mice were fed with standard chow or WD for 12 weeks.
(A-B) Plasma and hepatic bile acid levels (A), and gallbladder volume, biliary bile acid concentrations, and biliary bile acid content (B) (n = 6-8).
(C) Bile acid pool size in liver plus gallbladder (L+G), intestine and whole body (Total) (n = 5).
(D) Bile acid composition in the bile from gallbladder. Left, percentile compositionof different bile acids in the bile from flox/flox and BaLKO mice; Right,concentrations of four major bile acids in the bile. T-β-MCA, tauro-β-muricholicacid; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; CA, cholicacid; β-MCA, β-muricholic acid; α-MCA, α-muricholic acid; TLCA, taurolithocholicacid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid.
(E) Concentrations of total cholesterol (TC), free cholesterol (FC), andcholesterol ester (CE) in livers from control (dark bars) and BaLKO (gray bars)mice (n = 6-9).
(F) Plasma cholesterol levels in mice under fed and fasted conditions (n = 6).
(G) Food intake and fecal cholesterol content in WD-fed control and BaLKO mice (n = 4-5).
(H) Cholesterol uptake into plasma and liver following oral gavage of 3H-cholesterol (3H-chol.) for 2 h.
(I) Plasma cholesterol concentrations in WD-fed control and BaLKO mice treated with vehicle alone (CTR) or ezetimibe (EZ) for 4 days (n = 6); *P < 0.05 vs. flox/flox mice treated with vehicle alone; ns, not significant. In all panels, values are mean ± s.e.m.; *P < 0.05 by two-tailed Student's t-test.
