Abstract
Pericytes are mural cell that have been found to play important roles in promoting blood vessel development and regulating blood flow. The signals that attract pericytes to maturing vessels during the resolution phase of wound healing are unknown. In this study, we examine the role of the chemokine receptor CXC receptor 3 (CXCR3) ligands, as they are produced by maturing endothelial cells. Pericytes isolated from muscle and retina were found to by and large only express the B-isoform of CXCR3 (CXCR3B), with expression being independent of the mitotic state of the cells. Pericyte stimulation with the CXCR3 ligands Mig (CXCL9), IP-9/I-TAC (CXCL11) or IP-10 (CXCL10) resulted in the activation of ERK but not AKT. Treatment with Mig or IP-9, but not IP-10, enhanced p38MAPK phosphorylation. Interestingly, while cAMP is generated downstream of CXCR3B in other cells, PKA activation was not observed in these pericytes when treated with these three CXCR3 ligands. The increase in ERK activity resulted in a slight increase in cell transmigration, with the inhibition of ERK leading to a decrease in CXCR3B mediated migration and inhibition of p38MAPK reducing transmigration through small pores. These ligands did not affect proliferation. These data are the first to characterize CXCR3B as the predominant isoform expressed on pericytes, and was found on these diverse cells isolated from both muscle and eye. We also show that CXCR3B signaling stimulates transmigration of barrier pores in pericytes as opposed to its inhibitory affects on endothelial cells and fibroblasts. These findings characterize a novel role for the B-isoform of CXCR3 in regulating cellular function. Taken together these data demonstrate a role for CXCR3B in regulating pericyte function.
Keywords: pericytes, CXC receptor 3, Erk, AKT, p38MAPK, migration
Introduction
Angiogenesis and subsequent stabilization of the new vessels are critical to wound healing. The failure to establish and maintain functional vasculature leads to “failure to heal”. These nascent vessels are thought to be stabilized when coated by pericytes (1). Pericytes are vascular mural cells that are found at the abluminal surface of endothelial cell of capillaries, arterioles and venules (2). Although their function is not well understood, pericytes have been found to regulate capillary diameter and blood flow (3, 4), and vessel permeability and stabilization (1). The origin(s) of pericytes is not well defined, and may be myriad, since they have been found to derive from a variety of cells (5, 6). Furthermore pericytes are also thought to be multipotent, able to differentiate into a variety of cell types (7–9). However, the signals that drive pericyte function are poorly described and likely to be tissue- and process-specific.
Pericytes have been implicated in promoting would healing through their interaction with inflammatory cells (8, 10), endothelial cells (8, 11) and stromal cells (8, 12). However, during wound healing the main function of pericytes is considered to involve vascular development and maturation (2, 13). During wound repair initiation of angiogenesis involves the collapse of the vessels at the edge of the wound bed, with subsequent outgrowth of the endothelial cells as new, non- or poorly-functional vessels. These vessels are devoid of pericytes, as colocalization is not found until the resolving phase (14). The rapid outgrowth of immature vessels is followed by `pruning' of most of them during the resolving phase. Nearby pericytes are at least in part responsible for this involution by secreting CXCR3 ligands that triggers de-adhesion and endothelial cell anoikis (14). This is accomplished secondary to triggering of PLCβ driving a calcium flux that activates μ-calpain (CAPN1) to cleave integrin beta3 (15). How the pericytes then migrate to and stabilize the few remaining vessels is unknown, but likely critical to their persistence. Understanding the biology of these cells may provide a greater understanding of vessel maturation and may provide novel strategies for the improvement of wound repair and the treatment of certain vascular diseases.
Concomitant with pericyte coverage of a few dermal vessels is the expression of ligands for the CXCR3 receptor. As the wound bed matures, the ELR-negative chemokines CXCL10 (IP-10) and CXCL11 (IP-9/I-TAC) are expressed by redifferentiated keratinocytes and endothelial cells in maturing blood vessels (16). These chemokines promote the continuing reepithelialization by enhancing migration of dedifferentiated keratinocytes (17, 18). Later in the transition from granulation to resolving phase CXCL10 and CXCL11 inhibit endothelial and fibroblast in-migration (19–21), and actually induced endothelial cell anoikis (15). These functions of the ELR-negative CXC chemokines are distinct from the modulation of the innate immunity noted during the early inflammatory phase of healing (22). This is the milieu in which pericytes colocalize with and stabilize the maturing vessels. How these chemokines regulate pericyte function is not well defined even though they act at the time and location that pericytes are considered crucial to vessel maturation.
The common receptor for these ELR-negative chemokines, CXCR3, has been found to exist as two alternatively spliced forms designated CXCR3A and CXCR3B. Ligandation of the receptor on leukocytes, that overwhelmingly express the A-isoform, increases cell migration and proliferation (23–25). Binding to the B-isoform, the only isoform expressed on endothelial cells and fibroblasts, is inhibitory to locomotion (15, 19, 20, 26). CXCR3 has been reported to be expressed on mesangial and stellate cells, which are designated as pericytes of the kidney and liver, respectively, but the isoform expressed was not determined (27). Treatment of these cells with CXCL10 enhanced transmigration in mesangial and stellate cells but induced proliferation only in mesangial cells (27). These data suggest that CXCR3 signaling has a different spectrum of signaling to elicit different biological actions in a cell-specific manner in response to local events. In this study, we query what affects these wound `stop' signals would have on pericytes, using those isolated from both muscle and eye. We found that these express only the CXCR3 B-isoform, but do transmigrate in response to ligand, albeit at only a fraction of growth factor-induced relocalization. This occurs at least in part through activation of MAPK pathways. These findings demonstrate that activation of the CXCR3B signaling pathways can both promote and inhibit a cellular function and these responses are cell type specific.
Materials and Methods
Cells and cell culture
Primary human pericytes isolated from muscle (28) were a kind gift from Dr. Bruno Peaúlt (University of Pittsburgh/UCLA). The pericytes were flow sorted (CD146+/CD34−/CD45−/CD56−) and stained for αSMA, desmin and NG2 at passage 12 showing consistent expression of pericyte markers (14). The cells were grown in DMEM with high glucose (Invitrogen) supplemented with 20% FBS and 1% penicillin-streptomycin. Pericyte cultures between passages 9–14 were used for all experiments. Retinal pericytes were purchased from Cell Systems (Kirkland, WA) and grown in CSC complete classic medium with culture boost-R (Cell Systems). All cells were cultured on plastic at 37°C and 5% CO2.
RT-PCR of CXCR3
Total RNA was isolated from pericytes using the RNAeasy Kit (Qiagen) according to manufacture's protocol. cDNA synthesis was preformed using the Affinity Script cDNA Synthesis kit (Stratagene) according to manufacture's protocol. The cDNA was probed for CXCR3A and CXCR3B isoforms. The primers used for amplification of pan-CXCR3 was forward: 5'-AGCTTGACCGCTACCTGAA-3', reverse:5'-CGGAAACTTGACCCCTACAAA-3', CXCR3A was forward: 5'-AACCACAAGCACCAAAGCAG-3', reverse: 5'-TGATGTTGAAGAGGGCACCT-3', CXCR3B was forward: 5'-GCTGCTCAGAGTAAATCACAGACTA-3', reverse: 5'-TGATGTTGAAGAGGGCACCT-3', GAPDH was forward: 5'-ACCACAGTCCATGCCATCAC-3', reverse: 5'-TCCACCACCCTGTTGCTGTA-3'. The samples were cycled under the following conditions; 95°C 5 min, (94°C 30 sec, 53°C 30 sec, 72°C 1 min) 28x, 72°C 10 min, 4°C hold. The samples were diluted with 6x gel running dye () and run on a 1.5% agarose-TAE gel at 50 V for 90 min. The PCR products were stained with ethidum bromide (Sigma) and visualized using UV light. The bands were images using a Cannon PowerShot A720IS camera attached to a PhotoDoc-it imaging hood (UVP).
Migration assay
Cell migration was performed using an in vitro scratch assay as previously described (20). In brief, pericytes were plated at 1.0 × 105 cells/well in a 24 well TC plate and incubated at 37°C and 5% CO2 for 24 hrs. The cells were 100% confluent. Pericytes were resuspended in 0.1% dialyzed FBS-DMEM overnight at 37°C and 5% CO2. A denuded space was made in the center of the plate using a modified rubber policeman. The monolayer was scraped making a 1 mm wide denuded area. The cells were washed 2x with PBS then incubated in DMEM media supplemented with PDGF (50 ng/ml), PF4 (CXCL4), Mig (CXCL9), IP-10 (CXCL10), and IP-9 (ITAC/CXCL11) all at 100 ng/mL for 24 hrs at 37°C in 5% CO2. Images were taken at zero and 24 hrs, and the relative distance traveled by the cells into the acellular area was determined using MetaMorph. Cells treated with diluent were used at a control. Motility was determined by the decrease in the area of the denuded region. The change in area is represented as a percent of control.
Transmigration assay
Cells were analyzed for their ability to transmigrate on a complex bio-active matrix (Matrigel) using a transwell system (EMD Millipore). Transwells (24 well, 8.0 μm) were coated with Matrigel and allowed to dry. The Matrigel was rehydrated with 0.1% dialyzed FBS-DMEM (Gibco) at room temperature for 1.5 hrs. Pericytes were resuspended in 0.1% dialyzed FBS-DMEM (5.0 × 105 cells/ml), incubated for 15 min at room temperature then 200 μl were added to the upper chamber. To the lower chamber was added 800 μl of 0.1% dialyzed FBS-DMEM with Mig (CXCL9), IP-10 (CXCL10) or IP-9 (CXCL11) alone or in combination with a Mek inhibitor (PD980589, 10 μM) or p38MAPK inhibitor (SB203580, 10 μM). Cells were incubated for 48 hrs at 37°C in 5% CO2.
Protein Array assay
The angiogenesis (Angiogenesis Array C1000, Raybiotech Inc.) and chemokine (Chemokine Array C1, Raybiotech Inc.) arrays were performed according to manufactures protocol. In brief, pericytes, 95% confluent were incubated in 0.1% dialyzed FBS-DMEM overnight at 37°C in 5% CO2. The media was replaced with DMEM or DMEM supplemented with CXCL9, CXCL10 or CXCL11 (100 ng/ml) and incubated for 24 hrs at 37°C in 5% CO2. The supernatant was removed and centrifuged at 1500 rpm for 10 min to remove insoluble debris. The supernatant was analyzed for angiogenic factor and chemokine secretion.
Proliferation Assay
Analysis of cell proliferation was preformed using the MTS assay (Promega). Assay was preformed according to manufactures protocol. In brief, pericytes (MG-71 and MG-136) were incubated in 20% FBS, 1% Pen/Strep DMEM with high glucose and L-glutamine and without sodium pyruvate and HEPES (Life Technologies) until the cells were 90% confluent. The cells were washed with PBS then incubated with 0.1% dialyzed FBS-DMEM for 24hr at 37°C and 5% CO2. The cells were washed with PBS then detached. The cells were resuspended in 0.2% FBS-DMEM to a concentration of 1.0 × 105 cells/ml, then treated with Mig (CXCL9) (100 ng/ml), IP-9 (CXCL11) (100 ng/ml), IP-10 (CXCL10) (100 ng/ml), bFGF (50 ng/ml) or EGF (10 nM) for 48 hrs at 37°C in 5% CO2. MTS/PMS solution (20 μl) was added to each will and incubated for 3 hrs at 37°C in 5% CO2. The reaction was stopped with the addition of 10%SDS (25 μl). The samples were analyzed for the amount of soluble formazan produced using a SpectraMax M2 plate reader at 490nm (Molecular Devices). All samples were preformed in quadruplicate. The mean of each sample was determined and graphed as a percent of control (no treatment, NT).
Western Blot
Cells were lysed in RIPA buffer containing PMSF (1 mM), aprotinin (0.08 U/ml) and leupeptin (0.25 mg/ml). The lysate was sonicated then centrifuged to remove insoluble material. The lysate was diluted with 3x Laemmli buffer with 4% 2-mercaptoethanol. Proteins were separated by 10% SDS-PAGE. The proteins were transferred to PVDF membrane and immunoblotted using a rabbit anti-CXCR3 (Imgenex, San Diego, CA), rabbit anti-AKT (Cell Signailng), rabbit anti-pAKT (Cell Signaling), rabbit anti-MEK (Cell Signaling), rabbit anti-pMEK (Cell Signaling), rabbit anti-p38MAPK (Cell Signaling), rabbit anti-pp38MAPK (Cell Signaling) and anti-GAPDH (Abcam). The proteins were visualized using a secondary antibody IRDye 800CW-conjugated (LI-COR Biotechnology, Lincoln, NE) and visualized using an Odyssey CLx imager with Image Studio (LI-COR Biotechnology, Lincoln, NE).
Immunofluorescence
Pericytes were grown on gelatin coated chamber slides (Nalgene) for 24–48 hrs. Identification of pericytes markers was preformed using the following antibodies; anti-α-SMA (sigma), anti-NG2 (Milipore), anti-desmin (Cell Signaling), anti-CXCR3 (Abcam), and anti-CXCR3B (ProteinTech). For analysis of CXCL3 ligand secretion, pericytes were incubated in complete media with INF-γ (25 nM) for 4 hrs. Control cells were treated with an equal volume of diluent. The cells were fixed with 2% paraformaldehyde-PBS and then incubated with rabbit anti-IP-10 (PeproTech), rabbit anti-IP-9 (PeproTech) or IgG (Jackson Immunoresearch) antibody. The cells were incubated with Dapi (Sigma, St. Louis, MO) and a corresponding secondary antibody FITC-conjugated (Jackson Immunoresearch). The cells were visualized using an Olympus Fluoview 1000 confocal microscope using an UPlanSApo 20x/0.85 oil or UPlanFLN 40x/1.3 oil objective.
PKA Assay
Pericytes were grown on plastic in complete media until 85% confluent. The media was removed and replaced with 1% FBS-DMEM and incubated for 15 hrs. The media was removed and replaced with DMEM containing IP-9 (200 ng/ml) or IP-10 (200 ng/ml) and incubated for various times as indicated in the text. The cells were washed 1x with PBS pH 7.4 then lysed with PKA dissociation buffer. PKA activity was analyzed using a cAMP protein dependent Kinase assay kit (Promega) according to manufacture's protocol. The samples were run on a 0.8% agarose-Tris (50 mM, pH 6.8) at 100 V for 20 min. Samples were visualized by UV light and imaged using a Cannon PowerShot A720IS camera attached to a PhotoDoc-it imaging hood (UVP).
Statistical Analysis
All quantitative experiments were performed at least three times. Western blot analysis was done in triplicate for each cell line. Analysis of protein density was preformed using Image Studio (LI-COR Biotechnology, Lincoln, NE). For the migration and proliferation assays each ligand was assayed three times in quadruplicate. Statistical differences between groups were determined using a 2-tailed Student's t-test (paired) with significance determined as P<0.05. Results are expressed as mean ± s.e.m.
Results
Pericytes express CXCR3
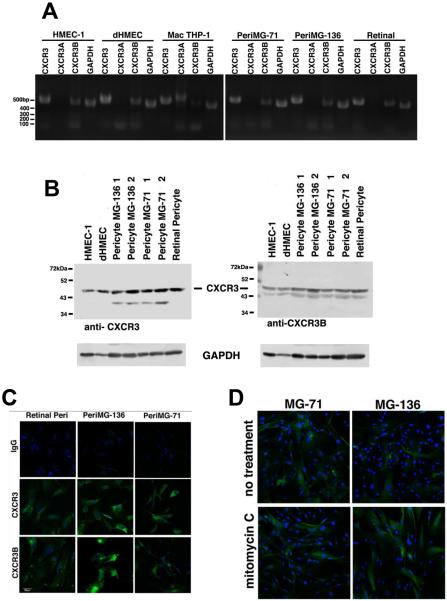
Hepatic stellate cells and glomerular mesangial cell, which are considered tissue-specific pericytes have been shown to express CXCR3 (29). We wanted to determine whether other pericytes, those isolated from muscle and the eye that are more often used in culture and regenerative manipulations, also expressed CXCR3. Using reverse transcriptase-PCR (rtPCR), we found that the muscle and retinal pericytes have significant expression of CXCR3B mRNA (Figure 1A). The expression of CXCR3A mRNA is absent or negligible (undetectable) in these pericyte cell lines, as validated by comparison to a CXCR3A-expressing cell, the primary human macrophage cell line, THP-1 (Figure 1A). Dermal microvascular endothelial cells HMEC-1 (immortalized) and dHMEC (primary) cells were use as controls since they only express CXCR3B. To determine that the CXCR3 B-isoform is presented as protein, immunoblotting and immunocytochemistry were performed (Figure 1B and 1C). Because no known antibody exists that specifically recognizes only the A-isoform of CXCR3 (CXCR3A), we are not able to directly determine whether pericytes present CXCR3A protein. However, when analyzing total CXCR3 protein using an antibody to the common sequences and comparing CXCR3 expression between endothelial cells and pericytes (Figure 1B, left blot), the pattern of expression was similar between endothelial cells and retinal pericytes. We also see roughly comparable staining between the endothelial cells and muscle pericytes (two different isolates, denoted as MG-136 and MG-71). Of interest, the muscle pericytes show a second protein band staining around 40 kDa, which may be a cleavage product. Since we do not detect CXCR3A mRNA (Figure 1A) this suggests that the lower molecular weight band is most likely a degradation product of CXCR3B. Taken together these data suggest that CXCR3B is the predominant or even only isoform expressed in pericytes isolated from the retina and muscle.
Figure 1. Pericytes express CXCR3 B-isoform.
Pericytes isolated from muscle and retina were analyzed for by PCR and Western blot and found to express CXCR3B. A.) mRNA from Pericytes from normal human muscle biopsy tissue (MG-71 and MG-136) and human retinal were analyzed by RT-PCR for CXCR3 expression. The CXCR3 primer set recognized both A- and B-isoforms, CXCR3A only recognizes the A-isoform and CXCR3B only recognizes the B-isoform. Primer sequences are listed in Materials and Methods. The results indicate that the pericytes only express CXCR3B. The human dermal endothelial cells (HMEC-1 and dHMEC) were used as positive controls for CXCR3B expression and the human macrophage cells (THP-1) were used as a positive control for CXCR3A expression. B.) Pericytes from muscle (MG-71 and MG-136) and human retinal were lysed in RIPA buffer and analyzed for CXCR3 using an antibody that recognizes both isoforms and an anti-CXCR3B antibody which only recognized the B-isoform. The 1 and 2 designation on MG-136 and MG-71 are for low passage (<8) and high passage (>12) respectively. The muscle and retinal pericytes express CXCR3B. C.) Pericytes were analyzed for surface expression of CXCR3. Pericytes were grown on gelatin coated glass slides, fixed the stained for CXCR3. The cells were not permeabilized and staining is representative of surface expression of CXCR3. D.) To determine whether CXCR3B expression is regulated by cell cycle, we incubated the cells with mitomycin C for 24 then stained for CXCR3. Pericytes from muscle and retina stained positive for surface CXCR3B indicating that CXCR3 is not entirely regulated by cell cycle. Shown are representative from at least two repeats of all experiments.
To bind ligands, CXCR3B must be accessible on the surface of pericytes. Pericytes were grown on collagen-coated glass slides and were fixed but not permeabilized before staining with monoclonal antibodies that recognize the extracellular domain of both CXCR3 isoforms (CXCR3) or only the B-isoform (CXCR3B) (Figure 1C). Both the retinal and muscle pericytes are shown to express CXCR3B on the cell surface under normal culture conditions (Figure 1C). It is well established that dermal microvascular endothelial cells only express CXCR3B (26). Thus, comparing CXCR3B expression between HMEC-1 and pericytes suggest that our muscle and retinal pericytes express similar levels of CXCR3B.
As CXCR3 expression is upregulated on microvascular endothelial cells during mitotic conditions and downregulated to essentially absent as the endothelial cells leave the cell cycle (G0 or senescence) (15, 20, 26), we wanted to determine whether CXCR3 is constitutively expressed on pericytes. Pericytes were analyzed for CXCR3 expression under mitotic and non-mitotic conditions. Incubation of the pericytes with mitomycin C for 48 hrs showed at least comparable expression of CXCR3 to mitotic cells grown in the absence of mitomycin C (Figure 1D). CXCR3 expression is observed to be ubiquitous regardless of mitotic potential. These data suggest that the predominant CXCR3 receptor expressed on pericytes isolated from muscle and retina is CXCR3B and expression is consistently present.
CXCR3 ligands activate MAPK pathways in pericytes
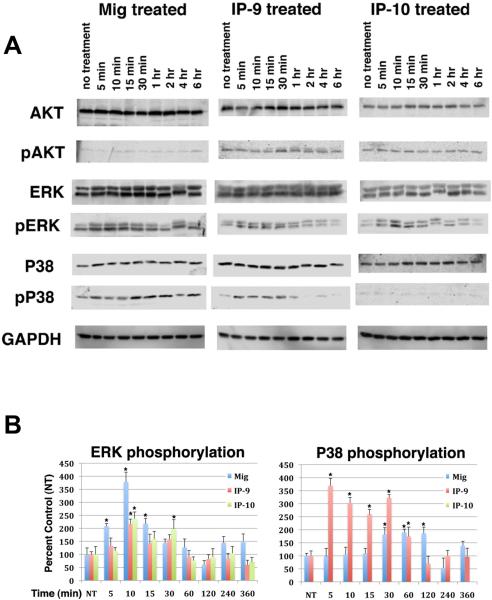
Ligandation of CXCR3 has been reported to activate the MAPK, AKT and PKA pathways in various cell types (20, 23, 26, 27, 30, 31). Here we sought to identify the signaling pathways activated by CXCR3 in pericytes. Treatment of pericytes with CXCL9 (Mig), CXCL10 (IP-10) or CXCL11 (IP-9) led to an increase in ERK phosphorylation that was initially observed within 5 min, peaked between 10–15 min and then was back to basal levels around 2 hrs (Figure 2, supplemental Figures 1, 2). Activation of CXCR3 was found to phosphorylate both ERK isoforms, p44ERK-1 and p42ERK-2, using an antibody that recognizes the phosphorylated forms of both isoforms. The data may suggest that CXCR3 activation leads to an increased activation of p42ERK-2, since there is an amplified staining of the 42 kDa band compared to the 44 kDa band (Figure 2).
Figure 2. ERK and p38MAPK are activated by CXCR3 ligands.
A.,) Pericytes (muscle, MG-136) were treated with CXCL9 (Mig), CXCL10 (IP-10) and CXCL11 (IP-9) at 100 ng/ml for various lengths of time. The cells were lysed in RIPA buffer and analyzed for total (AKT, ERK, p38) and phosphorylated AKT, ERK and p38MAPK (pAKT, pERK, pP38). Treatment of pericytes with the CXCR3 ligands induced phosphorylation of ERK and P38MAPK but did not induce phosphorylation of AKT. Shown are representative from at least three repeats of all experiments (n=3). B.) Protein band density of three independent experiments was analyzed using Image Studio 5.0 software (LI-COR, Lincoln, NE). Phosphorylation of the proteins is represented as a percentage of diluent treated (NT) cells. The graphs are an average of the individual experiments + s.e.m (n=3). * P < 0.05 comparing NT to CXCR3 ligand treated.
Activation of AKT was not observed for any of the three CXCR3 ligands (Figure 2, supplemental Figures 1, 2). Of interest was activation of p38MAPK. We show that stimulation of pericytes (MG-136, MG-71 and retinal) with CXCL9 (Mig) or CXCL11 (IP-9) but not CXCL10 (IP-10) enhanced p38MAPK phosphorylation (Figure 2B, supplemental 1B, 2B). Activation of p38MAPK in endothelial cells has been found to promote apoptosis (32) but has not been observed in pericytes. Previous work has identified distinct ligand binding regions on CXCR3 and the individual ligands can elicit disparate signaling pathways (33–35). Thus, the secretion of specific CXCR3 ligands at different phases of wound healing may impart different responses on pericyte function. Therefore pericyte function during wound healing may be differentially regulated at different stages of wound healing through the secretion of specific CXCR3 ligands.
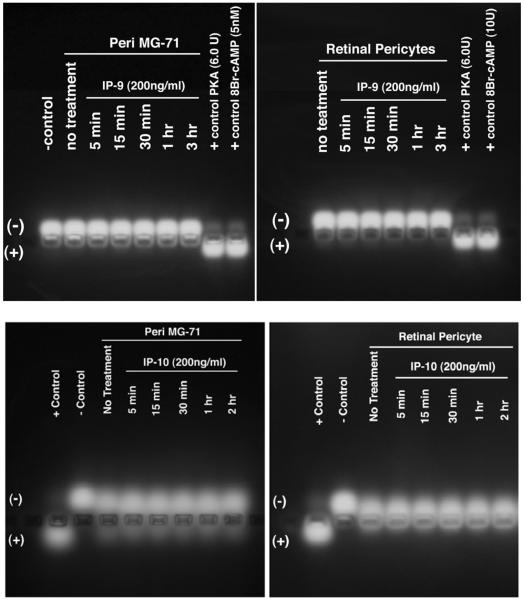
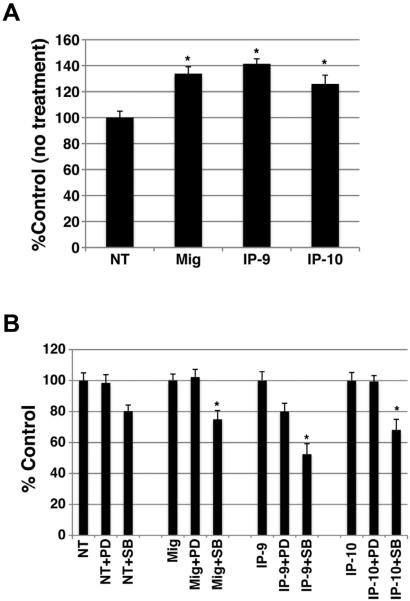
In endothelial cells and fibroblasts, CXCR3 abrogation of motility requires activation of PKA to phosphorylate m-calpain (CAPN) on ST369/370 and prevent rear detachment (20, 36). Therefore we queried whether PKA was activated by CXCR3 in pericytes. Pericytes were treated with CXCL10 (IP-10) or CXCL11 (IP-9) at 100 ng/ml for various lengths of time. The cells were lysed and then analyzed for PKA activity. Activation of PKA was not observed when the pericytes were stimulated with either of the CXCR3 ligands (Figure 3).
Figure 3. CXCR3 ligand do not activate PKA.
Pericytes were treated with IP-9 (CXCL11) or IP-10 (CXCL10) (200 ng/ml) for various length of time. The cells were lysed in PKA lysis buffer. The lysate was incubated with a synthetic peptide that was tagged. PKA activity is identified by phosphorylation of the peptide. Phosphorylation was identified by peptide migration to the anode. Incubation of pericytes with either IP-9 or IP-10 did not promote the activation of PKA. Shown is one of two independent experiments with similar results.
Proliferation is not affected by CXCR3 ligand stimulation
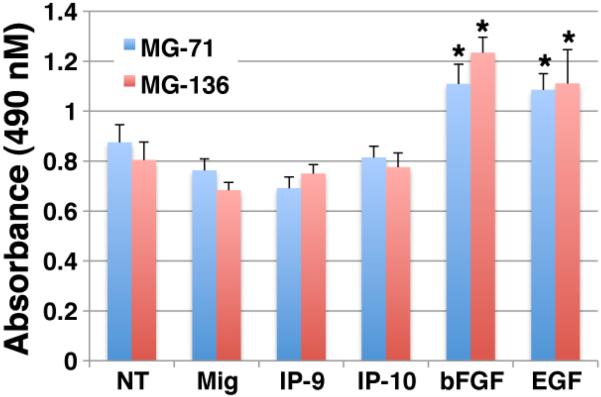
Angiogenesis requires endothelial cell proliferation, which then is shut down as the vessels mature and pericytes stabilize them. Additionally, the pericytes that are lost in the wound, require replacement likely from proliferation of existing pericytes in the peri-wound area, either prior to or after colocaliation with these nascent vessels. Since p38MAPK and Erk, that are activated in these cells, have been found to be involved in regulating proliferation, we analyze whether stimulation of pericytes with the CXCR3 ligands modulated cellular proliferation. Analysis of cell proliferation was performed using the MTS assay. Pericytes (MG-71 and MG-136) were plated on plastic and grown to 90% confluency then incubated in 0.2% dialyzed FBS-DMEM for 24 hrs to induce cell quiescence. The cells were detached and resuspended in 0.2% dialyzed FBS-DMEM media containing bFGF (50 ng/ml), EGF (10 nM), Mig (200 ng/ml), IP-9 (200 ng/ml) or IP-10 (200 ng/ml) and incubated for 48 hrs at 37°C, 5%CO2 then analyzed by the MTS assay. Our results show that treatment with CXCR3 ligands did not affect cell proliferation (Figure 4). Although, treatment with CXCL9 (Mig) and CXCL11 (IP-9) show a slight inhibition significance was not reached. The positive controls, bFGF or EGF treatment showed a significant increase in proliferation for both pericytes cell lines. These data suggest that CXCR3-triggered activation of Erk or p38MAPK does not regulate pericyte proliferation.
Figure 4. CXCR3 ligands do not induce proliferation.
Pericytes (muscle, MG-136) were quiesced in 0.2% dialyzed FBS-DMEM for 24hrs. The cells were incubated with CXCR3 ligands diluted in 0.2% dialyzed FBS-DMEM individually (100ng/ml final) for 48 hrs. The cell density was then measured by formazan production. The CXCR3 ligands do not induce proliferation. Shown are mean ± s.e.m of two experiments, each in triplicate, and each normalized to the cognate untreated controls. * P < 0.05 comparing NT to CXCR3 ligand treated.
CXCR3 ligands promote pericyte transmigration of barrier pores
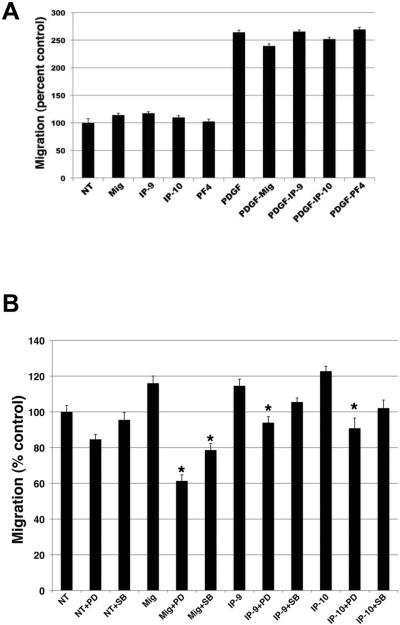
A second cell behavior required of pericytes during wound healing is in-migration into the wound bed and then association with maturing vessels. This requires both motility, that can be assessed by 2D migration, and ability to move through barrier pores, as assessed by translocation across pores smaller than cell size. It has been previously reported that activation of CXCR3 stimulated transmigration and proliferation of mesangial cells and smooth muscle cells (27, 37). Here we show when pericytes are treated with CXCR3 ligands, pericyte linear migration is slightly but reproducibly increased but the increase in migration did not reach statistical significance (Figure 5A). Migration was observed to be similar between treatments with all 3 CXCR3 ligands. When the cells were incubated with CXCL9, CXCL10, or CXCL11 in the presence of growth factors, the CXCR3 ligands did not have any additive effect on pericyte migration (Figure 5A).
Figure 5. CXCR3 ligands do not promote linear migration.
The scratch assay was used to determine 2D migration. Serum starved confluent pericytes (muscle, MG-136) were denuded and then incubated in CXCR3 ligands (100 ng/ml) only or with PDGF (50 ng/ml), SB203580 (10 μM) or PD98059 (10 μM) for 24hr. Migration of the cells into the denuded space was measured. A.) CXCR3 ligands may show a slight but reproducible enhancement of migration, though this did not rise to the level of statistical significance. B.) Inhibition of ERK (PD) showed a significant decrease in cell migration. Inhibition of P38MAPK (SB) showed only a modest inhibition of cell migration. These data indicate that CXCR3 activation of ERK mediates 2D migration. Shown are mean ± s.e.m of three experiments each in duplicate (n=3). * P < 0.05 comparing NT to CXCR3 ligand treated and untreated with inhibitor treatment.
Next, we tested whether activation of CXCR3 promoted transmigration of a fixed barrier; this is key to moving rapidly through barrier matrices such as the provisional matrix. Since transmigration requires the cells to perform different events compared to the scratch assay due to the necessity of the cell to degrade the Matrigel matrix and undergo deformation to migrate through the pores of the transwell, different signaling mechanism are required to promote this action. Pericytes were placed in the upper chamber of a transwell (8.0 μm) coated with Matrigel in 0.1% dialyzed FBS-DMEM media. The lower chamber contained 0.1% dialyzed FBS-DMEM media with either CXCL9, CXCL10, or CXCL11 at concentrations of 100 ng/ml. After 48 hrs the inserts were analyzed for the number of cells migrating through the insert. Treatment of pericytes with all 3 ligands promoted and increased in the transmigration of the pericytes (Figure 6A).
Figure 6. CXCR3 ligands promote transmigration.
Pericytes (muscle, MG-136) were analyzed for their ability to migrate through a Matrigel coated transwell (8.0 μm pore). Pericytes were suspended in 0.1% dialyzed FBS-media and plated in the upper chamber. The lower chamber contained 0.1% dialyzed FBS-media with CXCR3 ligands only or SB203580 (10 μM) or PD98059 (10 μM). The cells were incubated for 48 hrs A.) CXCR3 ligands enhance pericyte migration compared to diluent (NT). B.) Treatment of the cells with SB203580 (SB), P38MAPK inhibitor, significantly inhibited transmigration compared to PD98059 or diluent. These data suggest P38MAPK is involved in CXCR3 mediated transmigration. Shown are mean ± s.e.m of three experiments each in duplicate (n=3). * P < 0.05 comparing NT to CXCR3 ligand treated and untreated with inhibitor treatment.
We then determined whether activation of ERK or p38MAPK played a role in linear and trans-migration. It is well established that ERK activation promotes migration and p38MAPK activation has been linked to smooth muscle cells migration (38). Pericytes were preincubated with an ERK inhibitor (PD98059, 10 μM) or p38MAPK inhibitor (SB203580, 10 μM) for 1 hr. The cell monolayers were scratched with a modified rubber policeman washed with PBS then incubated with CXCL9 (Mig), CXCL10 (IP-10), or CXCL11 (IP-9) (final concentration 100 ng/ml) in the presence or absence of PD98059 or SB203580. Treatment of the cells with PD98059 significantly inhibited cell migration down below baseline in the presence of CXCR3 ligands (Figure 5B). Treatment with SB203580 showed only a modest decrease in migration (Figure 5B). Treatment with PD98059 did not inhibit pericyte transmigration for CXCL10 (IP-10) or CXCL11 (IP-9) but did show inhibition of CXCL9-stimulated pericyte transmigration (Figure 6B). On the other hand, SB203580 treatment showed a significant inhibition of transmigration for all treatments (Figure 6B). These results indicate that p38MAPK plays a role in pericyte transmigration of matrices driven by CXCR3 ligands and that ERK provides for linear migration likely affecting basal cell migration mainly.
Pericytes secrete CXCR3 ligands
Since very little is know regarding the role pericytes play in modulating wound healing, we want to identify whether stimulation of pericytes with CXCR3 ligands would modify protein secretion due to CXCR3 stimulation. Pericytes were incubated in 0.1% dialyzed FBS-DMEM for 18 hrs. The media was removed and then replaced with 0.1% dialyzed FBS-DMEM containing CXCL9, CXCL10, or CXCL11 (100 ng/ml) and incubated for 24hr. The conditioned media was removed and then centrifuged to remove debris. The supernatant was analyzed for chemokine and angiogenic factor secretion using a protein array ELISA (Ray Biotech Group). Treatment of the cells with the chemokines did not show an appreciable enhancement of chemokine secretion. Since we only looked at a small number of proteins, it is possible that chemokines not in our screen may be upregulated by CXCR3 ligand stimulation. Of interest, is that conditioned media from the unstimulated pericytes was positive for CXCL10 (IP-10) and CXCL11 (IP-9) (Supplemental Figure 3A). It is unknown whether CXCL9 (Mig) is secreted, since the array does contain this chemokine. These data may provide an explanation on why we only see a modest increase in pericytes migration when treated with CXCR3 ligands (Figure 5). The constitutive secretion of CXCR3 ligands would blunt the effects observed by the exogenous treatment. When the conditioned media was analyzed for the secretion of angiogenic factors, treatment of pericytes with the CXCR3 ligands did not show any considerable increase in any of the tested angiogenic factors (Supplemental Figure 3B). Of note, on the angiogenesis array C2 a significant increase in CXCL11 (IP-9) is observed in the CXCL11 treated conditioned media (Supplemental Figure 3B, Array C2 boxed proteins). This is a result of CXCL11 addition to the media prior to incubation with the cells.
Although CXCR3 activation by its ligands did not show any considerable increase in secretion of assayed proteins it does show that pericytes under normal culture condition constitutively secrete a number of factors. In the cytokine assay, in addition to CXCL10 (IP-10) and CXCL11 (IP-9) the pericytes were also found to have a high secretion of GRO, MIP-1β, RANTES and even higher secretion of MCP1. The antibody in this array recognizes all 3 GRO isoforms (GROα/CXCL1, GROβ/CXCL2 and GROγ/CXCL3). Since the array contains an antibody specific for GROα/CXCL1 and detection is very low indicates that GROβ and/or GROγ is being secreted. The receptor for GROβ and GROγ is CXCR2 and is expressed on monocytes/macrophage, neutrophil and epithelial cells and promote cell migration. MIP-1β (Macrophage inflammatory protein 1β) binds to CCR5 and promotes monocytes/macrophage migration. Both MCP-1 and RANTES promote angiogenesis. In the angiogenesis array C1, MCP-1, RANTES, ANG (Ang-1 and Ang-2), GRO, IL-6 and TIMP-1 and TIMP-2 are secreted. In the angiogenesis array C2, Ang-1, Ang-2 and MMP-1 are secreted. The increased MMP and TIMP species may provide for the enhanced transmigration of the Matrigel barrier in the transwell chambers. These data indicate that pericytes may play a role in homing of monocytes to the damaged tissue and promoting inflammation. This provides further data that pericytes promote angiogenesis and have the ability to inhibition new vessel growth and promoting the regression of nascent blood vessels as we have already shown (14).
Discussion
The events surround wound resolution are under-studied. Cellular events that drive the reversion from a highly cellular and vascularized dermis to a quiescent, pauci-cellular and almost avascular matrix are only now being deciphered. One of the more dramatic aspects is a greater than five fold reduction in the number of small blood vessels. Our earlier findings (15, 16) highlight the roles of the CXCR3 ligands CXCL10 (IP-10) and CXCL11 (IP-9/I-TAC) in initiating endothelial anoikis and vascular involution. However, open questions abound as how the pericytes, that mainly function to stabilize vessels function in this milieu. This study represents a first, in vitro step in discerning the response of the pericytes to these CXCR3 ligands. Herein, we show that while the pericytes secrete CXCL10 and CXCL11, and express the CXCR3B receptor isoform, they show only a limited responsiveness to this `stop healing' signal. However, the cellular events initiated promote transmigration through a matrix, suggesting that the presence of these ligands might attract the pericytes into the resolving wound bed and towards the maturing vessels.
In this study we have characterized the CXCR3 isoform and the downstream signaling molecules of CXCR3 in pericytes isolated from muscle and retina, the two most commonly used sources of pericytes. Here we report, for the first time, that both muscle and retinal pericytes by and large express only the B-isoform of CXCR3. We also report that stimulation of CXCR3 by its ligands CXCL9, CXCL10 and CXCL11 activate ERK and p38MAPK to promote pericyte transmigration of a barrier matrix. Previous studies by our group and others have shown that activation of CXCR3B in endothelial cells inhibits the migratory response and promotes apoptosis (20, 26, 32). Whereas in hepatic stellate cells and mesangial cells, pericytes of the liver and kidney respectively, activation of CXCR3 was found to promote transmigration and proliferation (27). It has also been found that CXCR3 activation in smooth muscle cells promoted transmigration and proliferation through p38MAPK activation (38, 39). Our findings indicate that cell linage plays a key role in the signaling response mediated by CXCR3 and indicates that pericytes from different tissues may activate different signaling pathways as a response to local events associated with tissue injury.
Since pericytes are a very heterogenous population of cells that have been suggested to arise from both mesoderm and ectoderm origin it is possible that CXCR3 expression on pericytes is differentially regulated by location and/or origin and will require further investigation. Using PCR and Western blotting we show that CXCR3B is the predominant isoform expressed on retinal and muscle pericytes. Due to the lack of an antibody that specifically recognizes CXCR3A we are not able to definitively show that CXCR3A is not expressed on these cells but the lack of CXCR3A mRNA (Figure 1) is highly suggestive that CXCR3A protein expression is extremely low or absent from these cells. We further showed that CXCR3 was constitutively expressed on retinal and muscle pericytes (Figure 1) suggesting that CXCR3B expression on pericytes is not necessarily regulated by cell cycle as found in endothelial cells (26). Our findings show a novel function for CXCR3B in promoting chemotaxis. Previous studies by our group and others have shown that CXCR3B activation in endothelial cells inhibits chemotaxis (20, 26, 40, 41). Here we are the first to show that CXCR3B promotes cell migration through activation of ERK (Figure 5) and invasion through p38MAPK activation (Figure 6). The study by Bonacchi et al.(27) investigating the role of CXCR3 on stellate and mesangial cells, found CXCR3-dependent chemotaxis was a result of ERK activation, which is correlative with our findings as they used a similar transmigration assay. For the first time, we show herein that it is the ability to pass through a matrix and deform, rather than linear motility, that is increased. A divergent between our studies is their findings of AKT activation, which we do not observe. In addition, their study found CXCR3 ligands mediated proliferation in stellate cells but was not observed in our pericytes. In their study, they did not identify the CXCR3 isoform expressed by the stellate and mesangial cells and it should not be assume that the B-isoform of CXCR3 is expressed on these cells. The signaling differences found between the stellate, mesangial and our pericytes may be due to differential expression of CXCR3 isoforms between the cells.
We also sought to determine whether the activation of pericytes by CXCR3 altered its secretome of signaling factors. We queried 38 chemokines and 42 angiogenic factors at the protein level. Our results show that stimulation with the CXCR3 ligands did not enhance any of the chemokine or angiogenic above basal levels. Since we only investigated a small sample of all chemokines and angiogenic factors, the possibility exists that the CXCR3 ligands may enhance the production of other factors. What is shown by this data is that unstimulated pericyte seem to constitutively express CXCR3 ligands. We found that pericytes grown in culture have high levels of IP-9 and IP-10. Parenthetically, since CXCL9 was not on the array we do not know if it is also constitutively expressed. This data supports our previous finding indicating that pericytes are able to secrete CXCR3 ligands (14). Further studies are required to fully understand CXCR3 ligand regulation in pericyte function. Continued knowledge of CXCR3 signaling in pericytes will provide greater information on pericyte function during wound healing.
In conclusion, the data from this study provides novel information on the downstream signaling pathways and cell behaviors in pericytes after ligandation of the CXCR3B receptor. A picture is emerging of pericytes responding to these ligands, that appear during the transition to wound resolution, by increased transmigration through a barrier matrix and small pores (and possibly to a lesser extend, a slight increase in migration rate). This would promote movement into the maturing dermal matrix and association with the many vessels. For immature vessels, the pericytes likely aid in driving involution via their own production of CXCL10 (IP-10) and CXCL11 (IP-9) as shown earlier (14). However, for the minority of maturing small vessels, being resistant due to CXCR3 downregulation on the endothelial cells (14, 15), the pericytes would colocalize and stabilize these few remaining functional vessels. This foundational model now needs to be investigated in organismal wound healing models. In addition the effects of the pericyte-derived CXCR3 ligands on inflammation (23, 42) needs to be studied in whole animals. However, such extensive work lies beyond the scope of these initial findings of the molecular signaling pathways and cellular responses.
Supplementary Material
Acknowledgements
This work was supported by grants from NIH (GM63569, GM69668). We thank members of the Wells laboratory for constructive comments and suggestions. The VA Pittsburgh Health System provided support in-kind.
Footnotes
Neither of the authors have any conflicts of interest in terms of the materials included herein.
References
- 1.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Experimental Cell Research. 2006;312(5):623–9. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169(1):1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 3.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–4. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. Journal of Cell Biology. 2001;153(3):543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation Research. 2005;97(6):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 6.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110(15):2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 7.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol. 2012;44(11):1800–12. doi: 10.1016/j.biocel.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills SJ, Cowin AJ, Kaur P. Pericytes, mesenchymal stem cells and the wound healing process. Cells. 2013;2(3):621–34. doi: 10.3390/cells2030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu Z, Li Y, Smith DS, Sheibani N, Huang S, Kern T, et al. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52(12):9005–10. doi: 10.1167/iovs.11-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morikawa S, Ezaki T. Phenotypic changes and possible angiogenic roles of pericytes during wound healing in the mouse skin. Histol Histopathol. 2011;26(8):979–95. doi: 10.14670/HH-26.979. [DOI] [PubMed] [Google Scholar]
- 12.Juniantito V, Izawa T, Yuasa T, Ichikawa C, Yamamoto E, Kuwamura M, et al. Immunophenotypical analyses of myofibroblasts in rat excisional wound healing: possible transdifferentiation of blood vessel pericytes and perifollicular dermal sheath cells into myofibroblasts. Histol Histopathol. 2012;27(4):515–27. doi: 10.14670/HH-27.515. [DOI] [PubMed] [Google Scholar]
- 13.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;(94):115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 14.Bodnar RJ, Rodgers ME, Chen WC, Wells A. Pericyte regulation of vascular remodeling through the CXC receptor 3. Arterioscler Thromb Vasc Biol. 2013;33(12):2818–29. doi: 10.1161/ATVBAHA.113.302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar RJ, Yates CC, Rodgers ME, Du X, Wells A. IP-10 induces dissociation of newly formed blood vessels. Journal of Cell Science. 2009;122(Pt 12):2064–77. doi: 10.1242/jcs.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, et al. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. American Journal of Pathology. 2007;171(2):484–95. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satish L, Yager D, Wells A. Glu-Leu-Arg-negative CXC chemokine interferon gamma inducible protein-9 as a mediator of epidermal-dermal communication during wound repair.[see comment] Journal of Investigative Dermatology. 2003;120(6):1110–7. doi: 10.1046/j.1523-1747.2003.12230.x. [DOI] [PubMed] [Google Scholar]
- 18.Yates CC, Whaley D, Hooda S, Hebda PA, Bodnar RJ, Wells A. Delayed reepithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair & Regeneration. 2009;17(1):34–41. doi: 10.1111/j.1524-475X.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiraha H, Glading A, Gupta K, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146(1):243–54. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98(5):617–25. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huen AC, Wells A. The Beginning of the End: CXCR3 Signaling in Late-Stage Wound Healing. Adv Wound Care (New Rochelle) 2012;1(6):244–8. doi: 10.1089/wound.2011.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 23.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–31. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodge DL, Schill WB, Wang JM, Blanca I, Reynolds DA, Ortaldo JR, et al. IL-2 and IL-12 alter NK cell responsiveness to IFN-gamma-inducible protein 10 by down-regulating CXCR3 expression. Journal of Immunology. 2002;168(12):6090–8. doi: 10.4049/jimmunol.168.12.6090. [DOI] [PubMed] [Google Scholar]
- 25.Reckamp KL, Figlin RA, Moldawer N, Pantuck AJ, Belldegrun AS, Burdick MD, et al. Expression of CXCR3 on mononuclear cells and CXCR3 ligands in patients with metastatic renal cell carcinoma in response to systemic IL-2 therapy. Journal of Immunotherapy. 2007;30(4):417–24. doi: 10.1097/CJI.0b013e31802e089a. [DOI] [PubMed] [Google Scholar]
- 26.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197(11):1537–49. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, et al. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem. 2001;276(13):9945–54. doi: 10.1074/jbc.M010303200. [DOI] [PubMed] [Google Scholar]
- 28.Crisan M, Deasy B, Gavina M, Zheng B, Huard J, Lazzari L, et al. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods in Cell Biology. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- 29.Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. Journal of Experimental Medicine. 1998;187(1):129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta D, Banerjee P, Gasser M, Waaga-Gasser AM, Pal S. CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1. J Biol Chem. 2010;285(47):36842–8. doi: 10.1074/jbc.M110.170324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satish L, Blair HC, Glading A, Wells A. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Molecular & Cellular Biology. 2005;25(5):1922–41. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrai I, Romboutsa K, Lasagni L, Annunziato F, Cosmi L, Romanelli RG, et al. Activation of p38MAPK mediates the angiostatic effect of the chemokine receptor CXCR3-B. International Journal of Biochemistry & Cell Biology. 2008 doi: 10.1016/j.biocel.2008.01.008. in Press. [DOI] [PubMed] [Google Scholar]
- 33.Kouroumalis A, Nibbs RJ, Aptel H, Wright KL, Kolios G, Ward SG. The chemokines CXCL9, CXCL10, and CXCL11 differentially stimulate G alpha i-independent signaling and actin responses in human intestinal myofibroblasts. Journal of Immunology. 2005;175(8):5403–11. doi: 10.4049/jimmunol.175.8.5403. [DOI] [PubMed] [Google Scholar]
- 34.Trotta T, Costantini S, Colonna G. Modelling of the membrane receptor CXCR3 and its complexes with CXCL9, CXCL10 and CXCL11 chemokines: putative target for new drug design. Molecular Immunology. 2009;47(2–3):332–9. doi: 10.1016/j.molimm.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Xanthou G, Williams TJ, Pease JE. Molecular characterization of the chemokine receptor CXCR3: evidence for the involvement of distinct extracellular domains in a multi-step model of ligand binding and receptor activation. Eur J Immunol. 2003;33(10):2927–36. doi: 10.1002/eji.200324235. [DOI] [PubMed] [Google Scholar]
- 36.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22(8):2716–27. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Yue TL, Ohlstein EH, Sung CP, Feuerstein GZ. Interferon-inducible protein-10 involves vascular smooth muscle cell migration, proliferation, and inflammatory response. J Biol Chem. 1996;271(39):24286–93. doi: 10.1074/jbc.271.39.24286. [DOI] [PubMed] [Google Scholar]
- 38.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, et al. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. Journal of Biological Chemistry. 1999;274(34):24211–9. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Qiong Y, Gardner DG. A role for p38 mitogen-activated protein kinase and c-myc in endothelin-dependent rat aortic smooth muscle cell proliferation. Hypertension. 2006;47(2):252–8. doi: 10.1161/01.HYP.0000198424.93598.6b. [DOI] [PubMed] [Google Scholar]
- 40.Yates C, Rodgers M, Jaynes J, Wells A, Bodnar R, Turner T. An IP-10 Derived Peptide Inhibits Angiogenesis. PLoS One. 2012 doi: 10.1371/journal.pone.0040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struyf S, Salogni L, Burdick MD, Vandercappellen J, Gouwy M, Noppen S, et al. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood. 2011;117(2):480–8. doi: 10.1182/blood-2009-11-253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, et al. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med. 2011;208(8):1605–20. doi: 10.1084/jem.20102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.