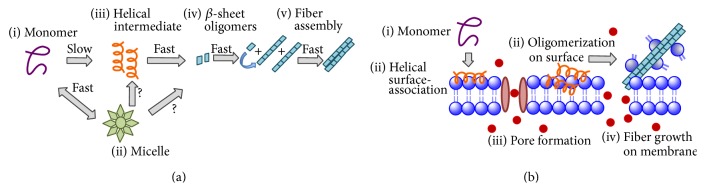
Figure 1.
Simplified representation of hIAPP aggregation in solution (a) and on the membrane (b). In solution (a), hIAPP initially exists in most experiments as a monomeric peptide (i) in exchange with a micelle-like form (ii). Transient helical (iii) and β-sheet (iv) intermediates have been proposed but the exact nature and order of these species in the aggregation process are not clear. Once the nucleus for aggregation is formed, the final steps of the aggregation process are the elongation of the fiber by the addition of monomers (or possibly β-sheet oligomers) to the ends of the fiber and the lateral association of individual fiber strands (protofilaments) to form the amyloid fiber (v). On the membrane (b), monomeric hIAPP (i) can bind the membrane (ii) and self-associate on the membrane to form pores (iii) or helical structures (iv) that eventually form membrane-associated amyloid fibers (iv). During the fiber formation process (iv), lipids can be incorporated into the hydrophobic ends of the fiber which would be otherwise exposed to water, causing disruption of the membrane (iv).