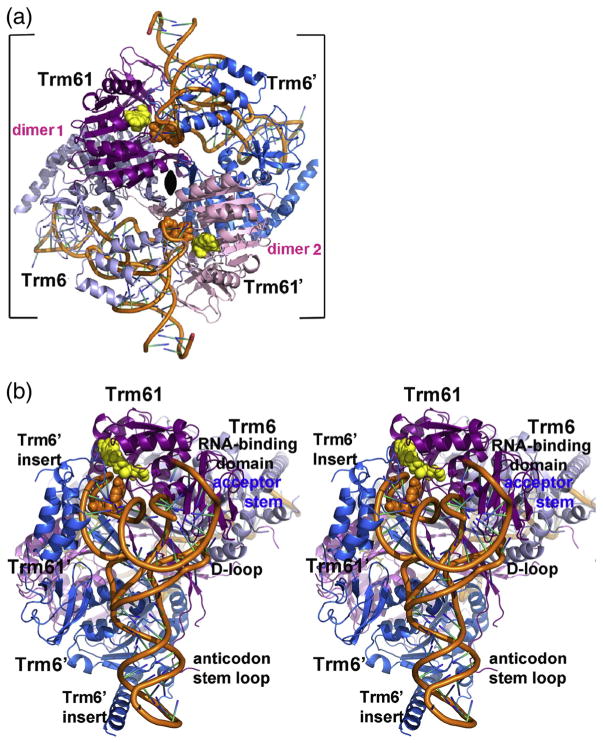
Fig. 2.
Quaternary structure of human m1A58 MTase. (a) Ribbon drawing of tRNA3 Lys-bound human m1A58 MTase heterotetramer viewed looking down the crystallographic 2-fold that coincides with the molecular 2-fold of the heterotetramer in the tetragonal crystal form. The 2-fold axis is indicated by the black oval. The tight dimers are colored magenta and gray-blue (“dimer 1”) and pink and blue (“dimer 2”). The tRNA-binding sites span the tight heterodimers: Trm61 and Trm6′ contribute 1522 Å2 and 1673 Å2, respectively, to the tRNA interface. The target nucleotide and the cofactor product SAH are shown in orange and yellow space-filling spheres, respectively. (b) Stereo plot of the heterotetramer viewed looking into the tRNA-binding site. The color scheme is the same as in (a). The view is related to the view in (a) by a 45° clockwise rotation about the crystallographic 2-fold and an approximately 90° rotation about the vertical axis in the plane of the paper.