Figure 3.
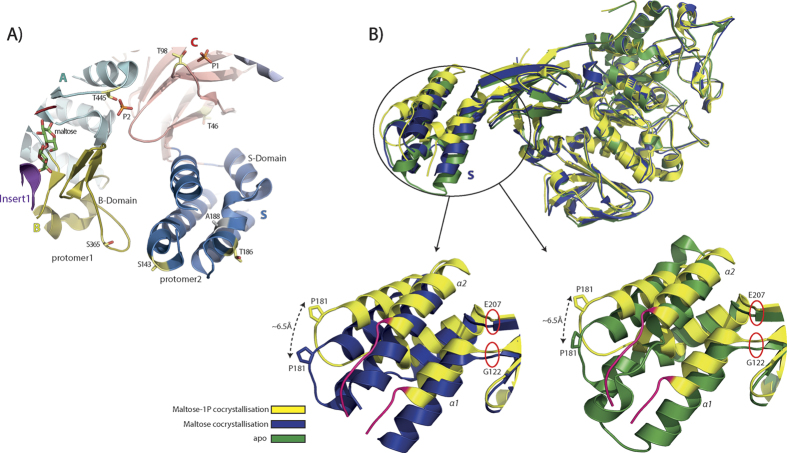
(A) View of M. tuberculosis GlgE phosphorylation sites mapped in M. thermoresistibile GlgE structure (5GCM). T46 and T186 are conserved in M. tuberculosis and M. thermoresistibile. S143 and S365 are mutated to threonine in M. tuberculosis. A188 and M80 are mutated to serine in M. tuberculosis. The side chain of S143 and the loop where M80 is located were not modeled since electron density is poor in those regions. The putative phosphorylation site T98 and T445 are also highlighted. (B) Superposition of structures of maltose co-crystallization (5GCM), maltose-1P co-crystallization (5ICM) and apo form (5CJ5). The represented protomer of maltose-1P structure has maltose bound. Regions that lose helical conformation in the maltose-1P co-crystallization structure are highlighted in red.