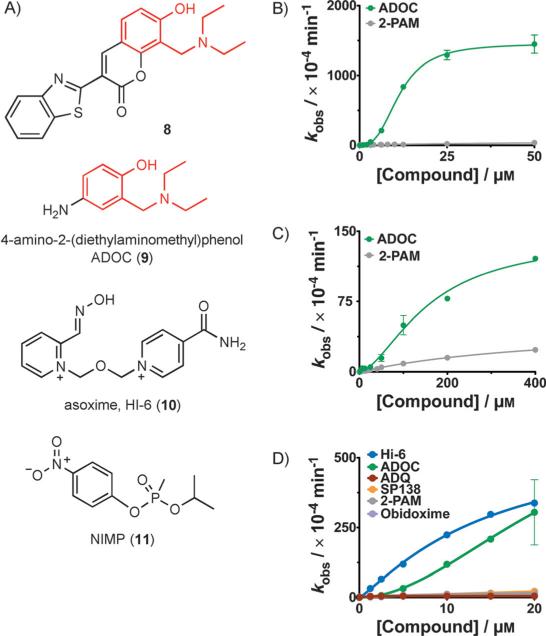
Figure 2.
Additional investigation into reactivation by these compounds. A) Structures are shown for: compound 8, an example of a compound containing a Mannich phenol that was identified to have significant reactivation activity, ADOC (9), the minimal functional group that is highly active, HI-6 (10), the oxime currently used as a therapeutic in Europe, and NIMP (11), a safer nerve agent analogue that leaves the same adduct as sarin (3) in the AChE active site. B), C) Concentration dependence of reactivation by 9 of B) 1-inhibited, or C) 2-inhibited human AChE. Reactivation by 9 is much faster than reactivation by pralidoxime (5, gray) and is best fit by a sigmoidal curve, indicative of cooperativity. D) Reactivation of the sterically hindered adducts left by 11 on huAChE. ADOC (9, green) is much more efficient than standard oximes pralidoxime (5, gray) and obidoxime (purple) and reaches the activity of HI-6 (10, blue).