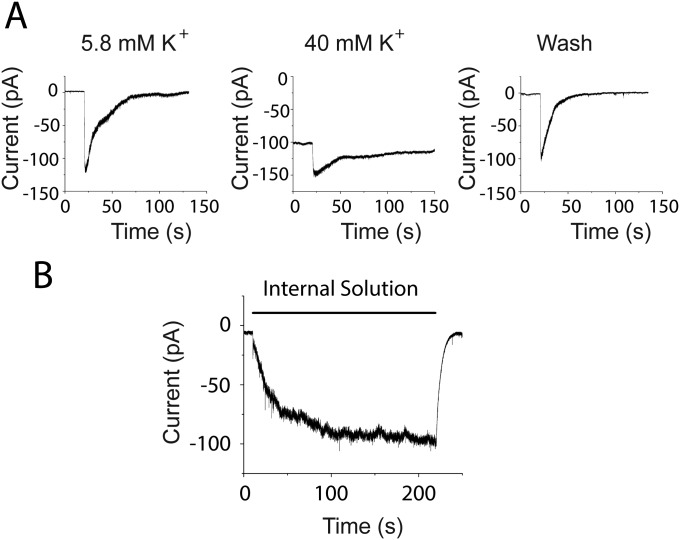
Fig. S2.
Potassium ions contribute to the peak damage-induced current. (A) External potassium was elevated from 5.8 to 40 mM by substitution with sodium, shifting the potassium equilibrium (EK) potential from −80 to −31 mV. Thus, the effect of any additional potassium released by hair cell damage will be reduced in this condition. In normal saline, hair cell rupture might expose nearby tissue to ∼150 mM K+, producing an inward current due to the change in EK from −80 to 0 mV. In the presence of 40 mM potassium, the same bolus of high potassium will change the driving force from −31 to 0 mV. Assuming the induced inward current is purely due to K+, the evoked current should be 2.6-fold smaller. The peak amplitude of the fast component was reversibly decreased 1.7-fold (from 94.1 ± 14.1 to 54.5 ± 5.9 pA; three experiments in three afferents) by prior exposure to 40 mM K+. Given that the actual changes in potassium concentration are unknown, this result supports the suggestion that the early inward current is carried at least in part by potassium ions. (B) Superfusion with “internal solution” (150 mM K-methanesulfonate, buffered calcium, no ATP) induced large inward currents in type II afferents. In 10 trials in three afferents, the maximum response induced by puffing internal solution averaged 97.4 ± 6.7 pA, not significantly different from the response induced by OHC ablation (P = 0.103), suggesting potassium release is able to depolarize type II afferents.