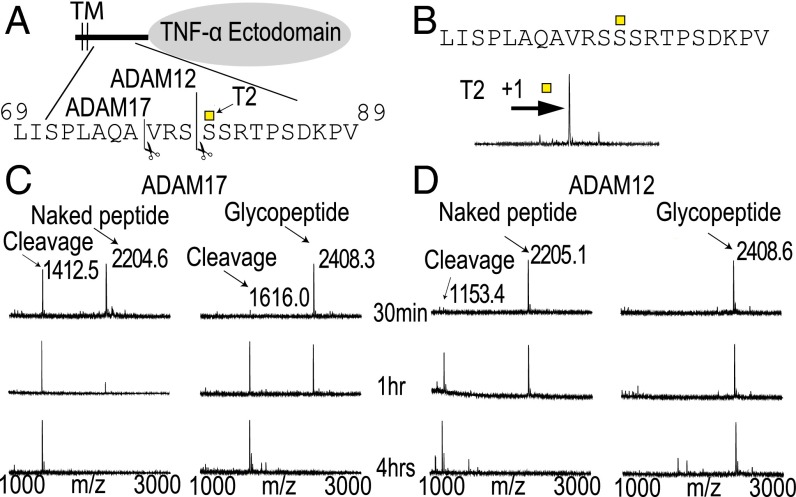
Fig. 3.
TNF-α is selectively O-glycosylated by GalNAc-T2, and this modulates in vitro ADAM cleavage. (A) Schematic drawing of TNF-ectodomain shedding zooming in on the juxtamembrane part that undergoes cleavage with indicated cleavage and glycosylation sites. (B) In vitro glycosylation of the juxtamembrane part of TNF-α with GalNAc-T2. Horizontal arrow indicates mass shift due to GalNAc glycosylation. (C) In vitro cleavage analysis of TNF-α peptide and glycopeptide by ADAM17 monitored by MALDI-TOF. Products formed after 30 min, 1 h, and 4 h are shown. ADAM17 cleaved at 76A↓V. Cleavage of the glycopeptide was less efficient. (D) ADAM12 cleaved at 79S↓S. Cleavage of the glycopeptide was almost completely abolished. ADAM9 and -10 had similar cleavage patterns as ADAM17 (Fig. 2). ADAM8 was not observed to cleave the peptide. Sequence, cleavage site (scissors), and site of glycosylation (square) are shown above each spectra.