Fig. 1.
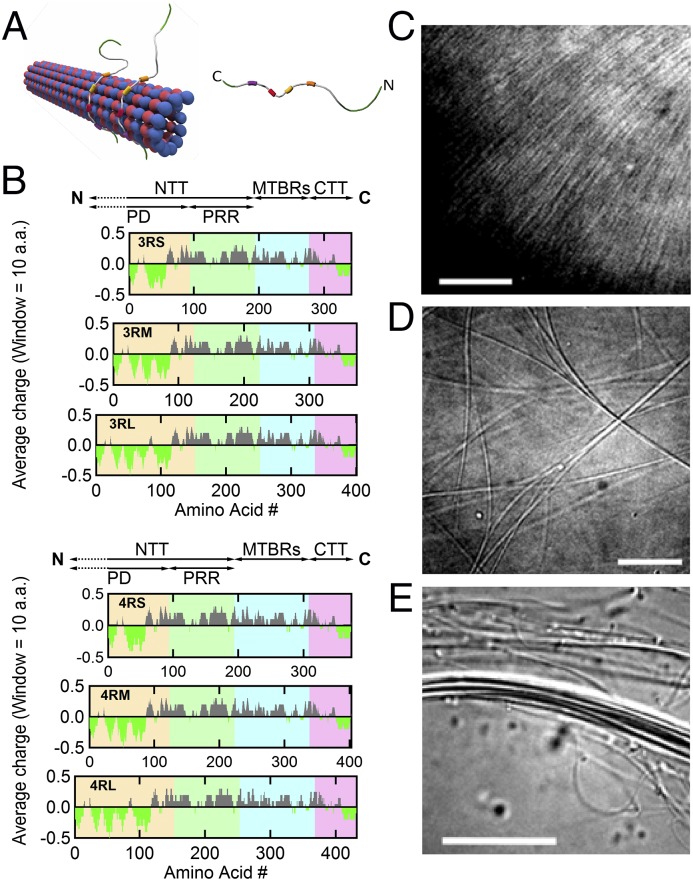
MT-associated protein Tau binds to the MT surface and modulates the higher-order structure of MTs with increasing osmotic pressure. (A) Schematics of (Left) an MT with bound Tau protein and (Right) a single Tau protein (labeled with N- and C-terminal ends) with four MT-binding repeats (colored boxes). (B) Charge (averaged over 10 residues) vs. amino acid residue number for six isoforms of human Tau. The charge distribution diagram of each isoform shows the cationic (gray) nature of Tau, with the exception of the amino- and carboxyl-terminal tails, which include anionic (bright green) regions that lead to dipole-like characteristics. Data were obtained from the National Center for Biotechnology Information Protein Database (accession nos. NP_058525.1, NP_001190180.1, NP_001190181.1, NP_058518.1, NP_001116539.1, and NP_005901.2 for 3RS, 3RM, 3RL, 4RS, 4RM, and 4RL, respectively). The N-terminal tail (NTT) is made up of the PD (yellow background; first 92, 121, and 150 amino acids for -S, -M, and -L isoforms, respectively) and PRR (green background; next 94 amino acids) followed by the MT-binding region (MTBR; blue background) and ending at the carboxyl terminus (C) with the C-terminal tail (CTT; pink background). Tau isoforms have either three or four MT-binding repeats (3R- or 4R-) as a result of excluding (or including) exon 10 (33 amino acids), which contains a second MT-binding repeat and the interrepeat region between the first and second repeats. Additionally, the exclusion of exons 2 and 3 (both 29 amino acids), the exclusion of exon 2, or no exclusions in the PD result in the short (-S), medium (-M), or long (-L) isoforms, respectively. (C–E) DIC microscopy of samples at Φ3RL = 1/40 show the presence of (C) an unbundled and (D and E) two distinct bundled phases at 0, 1, and 10 wt% 20k PEO, respectively. (Scale bars: C, 10 μm; D, 10 μm; E, 20 μm.)