Abstract
Intracellular calcium transients generated by activation of voltage-gated calcium (CaV) channels generate local signals, which initiate physiological processes such as secretion, synaptic transmission, and excitation-contraction coupling. Regulation of calcium entry through CaV channels is crucial for control of these physiological processes. In this article, I review experimental results that have emerged over several years showing that cardiac CaV1.2 channels form a local signaling complex, in which their proteolytically processed distal C-terminal domain, an A-Kinase Anchoring Protein, and cyclic AMP-dependent protein kinase (PKA) interact directly with the transmembrane core of the ion channel through the proximal C-terminal domain. This signaling complex is the substrate for β-adrenergic up-regulation of the CaV1.2 channel in the heart during the fight-or-flight response. Protein phosphorylation of two sites at the interface between the distal and proximal C-terminal domains contributes importantly to control of basal CaV1.2 channel activity, and phosphorylation of Ser1700 by PKA at that interface up-regulates CaV1.2 activity in response to β-adrenergic signaling. Thus, the intracellular C-terminal domain of CaV1.2 channels serves as a signaling platform, mediating beat-to-beat physiological regulation of channel activity and up-regulation by β-adrenergic signaling in the fight-or-flight response.
INTRODUCTION
Ca2+ channels in many different cell types activate upon membrane depolarization and mediate Ca2+ influx in response to action potentials and sub-threshold depolarizing signals. Ca2+ entering the cell through voltage-gated Ca2+ (CaV) channels serves as the second messenger of electrical signaling, initiating many different cellular events. In cardiac and smooth muscle cells, activation of Ca2+ channels initiates contraction directly by increasing cytosolic Ca2+ concentration and indirectly by activating calcium-dependent calcium release by ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum [1–4]. In skeletal muscle cells, voltage-gated Ca2+ channels in the transverse tubule membranes interact physically with ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum and activate them to initiate rapid contraction [5, 6]. The same Ca2+ channels in the transverse tubules also mediate a slow Ca2+ conductance that increases cytosolic concentration and thereby regulates the force of contraction in response to high-frequency trains of nerve impulses [5]. Ca2+ entering the cytosol via voltage-gated Ca2+ channels regulates enzyme activity, gene expression, and other biochemical processes [7].
Pioneering electrophysiological studies by Professor Harald Reuter first revealed the Ca2+ current in cardiac myocytes dissected from mammalian heart [8]. The limitations of voltage clamp equipment at the time made these studies difficult. Nevertheless, the general characteristics of voltage dependence, calcium selectivity, and kinetics of activation and inactivation of the cardiac Ca2+ current observed in these early studies have become hallmarks for Ca2+ channels studied over more than four decades [9–14]. Since those first recordings of Ca2+ currents in cardiac myocytes [1], it has become apparent that there are multiple types of Ca2+ currents as defined by physiological and pharmacological criteria [15–17]. In cardiac, smooth, and skeletal muscle, the major Ca2+ currents are distinguished by high voltage of activation, large single channel conductance, slow voltage-dependent inactivation, marked up-regulation by cAMP-dependent protein phosphorylation pathways, and specific inhibition by Ca2+ antagonist drugs including dihydropyridines, phenylalkylamines, and benzothiazepines [1, 15]. These Ca2+ currents have been designated L-type, as they have slow voltage-dependent inactivation and therefore are long-lasting when Ba2+ is the current carrier and there is no Ca2+-dependent inactivation [15].
The L-type calcium current in cardiac myocytes is the molecular target for PKA regulation of contraction in the fight-or-flight response, as shown in early work by Tsien, Greengard, and Reuter [18–20]. Activation of β-adrenergic receptors increases L-type Ca2+ currents through PKA–mediated phosphorylation of the Cav1.2 channel protein and/or associated proteins [18, 20–25]. The size of the L-type calcium current is tightly controlled by activation of PKA phosphorylation and dephosphorylation by phosphoprotein phosphatases [25, 26]. This dynamic regulation underlies the control of cardiac contractility on a beat-to-beat basis in the heart.
Modulation of ion channels is a dynamic process that is precisely controlled in space and time [27, 28]. Targeting and localization of signaling enzymes to discrete subcellular compartments or substrates is an important regulatory mechanism, ensuring specificity of signaling events in response to local stimuli [29]. In this article, I describe work on the signaling complexes formed by the skeletal and cardiac muscle calcium channels (CaV1.1 and CaV1.2) that initiate excitation-contraction coupling. In each case, signaling proteins and anchoring proteins that regulate these channels bind to specific sites on their intracellular C-terminal domains, and these protein-protein interactions are required for normal signal transduction in muscle cells. In the fight-or-flight response, a conserved physiological response of vertebrates to stress, fear, and exercise, both of these Ca2+ channel signaling complexes respond to local activation of cAMP signaling pathways and up-regulate calcium channel activity, leading to increased contractile force.
CALCIUM CHANNEL SIGNALING COMPLEXES IN SKELETAL AND CARDIAC MUSCLE
The Fight-or-Flight Response in Skeletal and Cardiac Muscle
To meet changing hemodynamic demands placed upon the heart, excitation-contraction coupling in cardiac myocytes is constantly modulated by multiple signaling pathways. The most important regulator of cardiac function on a beat-to-beat basis is the autonomic nervous system. Release of catecholamines from sympathetic nerve endings and the adrenal medulla stimulates β-adrenergic receptors in cardiac myocytes [3, 4, 30]. Stimulation of the β-adrenergic signaling pathway increases the chronotropic (heart rate), inotropic (strength of contraction during systole), and lusitropic (rate and extent of relaxation during diastole) states of the heart. At the cellular level, stimulation of β-adrenergic receptors activates PKA signaling pathways resulting in the phosphorylation of a number of target proteins, including voltage-gated calcium channels [31].
The force of contraction of skeletal muscle is also increased in the fight-or-flight response [32, 33]. Forceful contractions of skeletal muscle require tetanic bursts of action potentials generated in the motor nerve [34–36]. During β-adrenergic stimulation, the slowly activated calcium current in skeletal muscle is increased and this calcium entry is required for the increase in contractile force by treatment with adrenaline [37]. It is likely that the slowly activated calcium entry through voltage-gated calcium channels increases calcium in the cytosol, the sarcoplasmic reticulum calcium AT-Pase pumps that extra calcium into the sarcoplasmic reticulum and increases the calcium stores, and in subsequent contractions, the amount of calcium in the sarcoplasmic reticulum is greater and more calcium is released to activate actomyosin, resulting in an increase in contractile force.
A cAMP/PKA Signaling Complex formed by Skeletal Muscle Calcium Channels
Skeletal muscle Cav1.1 channels have been the primary experimental model for biochemical studies of calcium channels (Fig. 1A [38–40]). They are composed of a large pore-forming α1 subunit, a disulfide-linked glycoprotein complex of α2 and δ subunits, an intra-cellular β subunit, and a transmembrane, glycosylated γ subunit. Both the pore-forming α1 subunit and the auxiliary β subunit are phosphorylated by PKA [39–42]. The α1 subunit is truncated by proteolytic processing of the C-terminal domain [43, 44], and the primary sites of in vitro PKA phosphorylation are located in the distal C-terminus beyond the point of proteolytic cleavage [45, 46], suggesting that the distal C-terminal domain is not degraded but rather remains attached to the CaV1.1 channel and regulates it. Using mass spectrometric analysis of purified CaV1.1 channels, we found that the C-terminal domain of CaV1.1 channels is proteolytically processed at a specific site, Ala1664 [47]. The distal C-terminal domain truncated at Ala1664 binds to the proximal C-terminal domain in a specific complex [47], placing the anchored PKA and its primary sites of phosphorylation close to the proximal C-terminal domain and the remainder of the CaV1.1 channel.
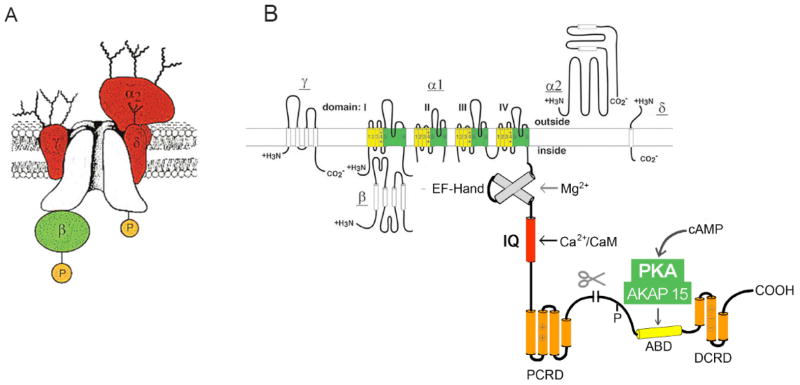
Fig. 1. The CaV1 calcium channel signaling complex.
A. Subunit structure of the CaV1.1 channel from skeletal muscle transverse tubules. The pore-forming α1 subunit is illustrated in the center of the complex with interacting β, α2δ, and γ subunits. Modified from [27, 40, 80]. B. A transmembrane folding diagram illustrating the subunits of CaV1.2 channels. The C-terminal domain of the cardiac calcium CaV1.2 channels is shown in expanded presentation to illustrate the regulatory interactions clearly. ABD, AKAP15 binding domain; DCRD, distal C-terminal regulatory domain; PCRD, proximal C-terminal regulatory domain; scissors, site of proteolytic processing. An EF-hand motif involved in regulation of Mg2+ and an IQ-like motif involved in calcium-dependent inactivation are also illustrated.
Voltage-dependent potentiation of Cav1.1 channels on the 50-msec time scale requires PKA phosphorylation [48] and PKA anchoring via an AKAP [49, 50], suggesting close association of PKA and Ca2+ channels. We found that a novel, plasma-membrane-targeted AKAP (AKAP15) is associated with Cav1.1 channels and may mediate their regulation by PKA [51, 52]. This AKAP is also known as AKAP18 [53]. AKAP15 binds to the distal C-terminal domain of Cav1.1 channels via a novel modified leucine-zipper interaction in the C-terminal domain near the primary sites of PKA phosphorylation [54]. Block of this interaction by competing peptides prevents PKA regulation of Ca2+ currents in intact skeletal myoblasts, indicating that direct interaction with the Cav1.1 channel is required for effective regulation in situ in skeletal muscle cells [54]. These results define a signaling complex of the transmembrane core of the Ca2+ channel, its proteolytically processed distal C-terminal domain, AKAP, and PKA that regulates skeletal muscle Ca2+ channels.
In order to identify the sites of PKA phosphorylation that may be involved in the fight-or-flight response in vivo, we used mass spectrometry to analyze the peptides in the intra-cellular domains of skeletal muscle CaV1.1 channels and identify their sites of in vivo phosphorylation [55]. This exhaustive analysis revealed two previously unidentified sites of in vivo phosphorylation, Ser1575 and Thr1579, which are strategically located at the interface between the distal C-terminal domain and the proximal C-terminal domain [55]. Ser1575 is in a classic consensus sequence for PKA phosphorylation, whereas Thr1579 is in a sequence compatible with phosphorylation by casein kinase II [55]. Using a phosphospecific antibody against Ser1575, we found that phosphorylation of this site in vivo is substantially increased by stimulation of rabbits by injection of the beta-adrenergic agonist isoproterenol and reduced by injection of beta-adrenergic antagonist propranolol [55]. These results strongly implicate phosphorylation of Ser1575 in regulation of the contractile force of skeletal muscle in response to stress, fear, and exercise.
A cAMP/PKA Signaling Complex Formed by Cardiac Ca2+ Channels
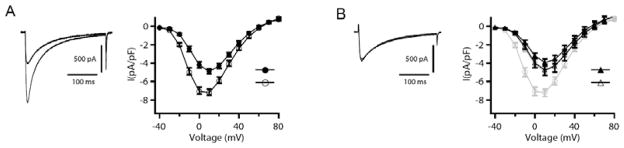
In cardiac myocytes, Ca2+ influx through Cav1.2 channels contributes to the plateau phase of the cardiac action potential and is responsible for initiating excitation-contraction coupling. Cav1.2 channels are modulated by the β-adrenergic receptor/cAMP signaling pathway [4, 30]. As for skeletal muscle Cav1.1 channels, both the pore-forming α1 and auxiliary β subunits of Cav1.2 channels are substrates for phosphorylation by PKA [56–59]. PKA phosphorylates the purified α1 subunit in vitro on a single site containing Ser1928 in the C-terminal domain [57, 60]. Activation of β-adrenergic receptors increases L-type Ca2+ currents through PKA–mediated phosphorylation of the Cav1.2 channel protein and/or associated proteins [18, 20–22]. As for skeletal muscle Cav1.1 channels, PKA regulation of Cav1.2 channels in dissociated cardiac myocytes is rapid and increases the level of current by 2- to 3-fold (Fig. 2A). This up-regulation of CaV1.2 channel activity in dissociated ventricular myocytes requires an AKAP bound to the AKAP binding site in the distal C-terminal domain, because it is blocked by specific peptides derived from that site (Fig. 2B [61]). Intracellular dialysis of ‘kinase-anchoring inhibitor peptides’, which competitively inhibit PKA-AKAP interactions, also effectively inhibit PKA-dependent increases of Ca2+ channel activity in cardiac myocytes [61]. These results suggest that a Ca2+ channel signaling complex containing an AKAP and PKA bound to the distal C-terminal domain is formed in cardiac muscle, as in skeletal muscle. Remarkably, even though there is a high concentration of PKA in the cardiac myocytes, only PKA specifically anchored to CaV1.2 channels can effectively regulate channel activity.
Fig. 2. Disruption of the AKAP15-leucine zipper interaction inhibits β-adrenergic receptor regulation of CaV1.2 channels in rat ventricular myocytes.
(A), Left panel, representative currents elicited by 300-ms test pulses to 0 mV before (closed symbols) and after (open symbols) 5 min exposure to Iso (1 μM). Right panel, mean (± sem) current-voltage relationships before (closed symbols) and after (open symbols) 5 min exposure to isoproterenol in the absence of peptide dialysis. (B), the effect of intracellular dialysis with AKAP15LZ (38–54) (100 μM; n = 14, black triangles) on the response of ICa to isoproterenol as compared to control (circles and gray). Modified from [61].
AKAPs that mediate PKA regulation of CaV1.2 channels
AKAP-15 is a prime candidate for the anchoring protein that targets PKA to Cav1.2 channels in cardiac muscle [61]. A combination of biochemical, site-directed mutagenesis, and electrophysiological studies identified a conserved leucine-zipper motif in the distal C-terminus of the pore-forming α1 subunit of Cav1.2 channels, which is required for the targeting of PKA and functional regulation by PKA signaling (Fig. 2 [61]). Mutation of this motif prevents PKA anchoring, and disruption of this interaction with competing peptides prevents β-AR- and PKA-dependent regulation of L-type calcium currents in ventricular myocytes [61]. Thus, PKA anchored directly to the Cav1.2 channel by AKAP15, or a related AKAP acting via a modified leucine-zipper interaction, is required for regulation of CaV1.2 channels in cardiac myocytes by the autonomic nervous system. Remarkably, block of kinase anchoring is as effective as block of kinase activity in preventing Cav1.2 channel regulation. Thus, this work provides evidence that PKA targeting to ion channels via leucine zipper interactions is absolutely required for regulation of Cav1.2 channels in intact myocytes.
Although studies of AKAP15 first focused attention on anchoring of PKA via an AKAP to the modified leucine motif on CaV1.2 channels, deletion of AKAP15 in mice does not prevent up-regulation of CaV1.2 channels by β-adrenergic stimulation at a saturating concentration (1 μM) of isoproterenol [62]. Mouse AKAP150 (designated AKAP79 in humans) also binds at the modified leucine zipper site on CaV1.2 channels [63, 64]. However, deletion of AKAP150 alone or both AKAP15 and AKAP150 in mice is not sufficient to prevent up-regulation of CaV1.2 channels in response to maximal stimulation with isoproterenol [62]. These results indicate that AKAP15 is not unique in its ability to up-regulate CaV1.2 channels and imply that an unidentified AKAP also is involved in this process. Further studies will be required to determine the full range of AKAPs that are able to support up-regulation of CaV1.2 channels in vitro and reveal which AKAPs are essential for that process in vivo.
CALCIUM CHANNEL REGULATION BY AN AUTOINHIBITORY SIGNALING COMPLEX
An autoinhibitory signaling complex formed by cardiac CaV1.2 channels
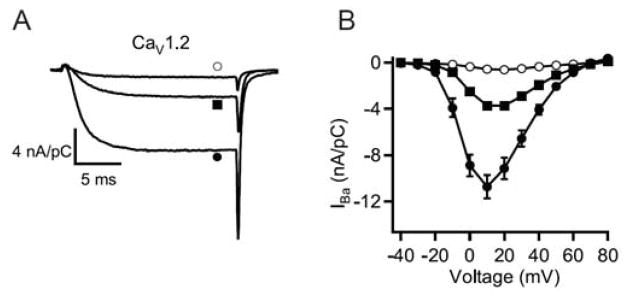
Although the distal C-terminal domain of CaV1.2 channels is proteolytically processed [57], binding of AKAP and PKA to this domain is required for regulation of these channels in intact cardiomyocytes [61]. These results imply that the distal C-terminal domain remains associated with the proteolytically processed cardiac CaV1.2 channel, and this is supported by evidence that the distal C-terminus can bind to the truncated CaV1.2 channel in vitro [65, 66]. Moreover, formation of this complex dramatically inhibits CaV1.2 channel function [67]. Deletion of the distal C-terminal near the site of proteolytic processing increases calcium channel activity (Fig. 3, square and filled circle [67, 68]). However, noncovalent association of the cleaved distal C-terminal reduces channel activity more than 10-fold, to a level much below that of channels with an intact C-terminus (Fig. 3, open circle [67]). These effects are caused primarily by reduction of the coupling of voltage sensing to channel opening, with an additional increase in channel activity from a shift of the voltage dependence of activation to more negative membrane potentials [67]. Thus, proteolytic processing produces an autoinhibited CaV1.2 channel complex containing noncovalently bound distal C-terminus with AKAP15 and PKA associated through a modified leucine zipper interaction. This autoinhibited complex may be the primary substrate for regulation of cardiac calcium channels by the β-adrenergic receptor/PKA pathway in vivo [67].
Fig. 3. Interaction of the cleaved distal C-terminus with truncated CaV1.2 channels and autoinhibition of channel activity.
(A) Representative Ba2+ currents elicited by 20-ms test pulses from −80 to +20 mV recorded through full-length CaV1.2 (closed squares), CaV1.2 Δ1821 (closed circles), and CaV1.2Δ1821 plus distal1822-2171 (open circles) channels. (B) Mean (± sem) current-voltage relationships of full-length CaV1.2 (closed squares), CaV1.2Δ1821 (closed circles), and CaV1.2Δ1821 plus distal1822-2171 (open circles) channels. Peak currents at +10 mV were: −3.73 ± 0.29 nA/pC (n=10) for full-length; −10.75 ± 1.07 nA/pC for CaV1.2Δ1821, n = 11, p<0.001; and −0.55 ± 0.08 nA/pC, n=24 for CaV1.2Δ1821 plus distal1822-2171. Modified from [67].
Reconstitution of PKA regulation of CaV1.2 channels in transfected nonmuscle cells
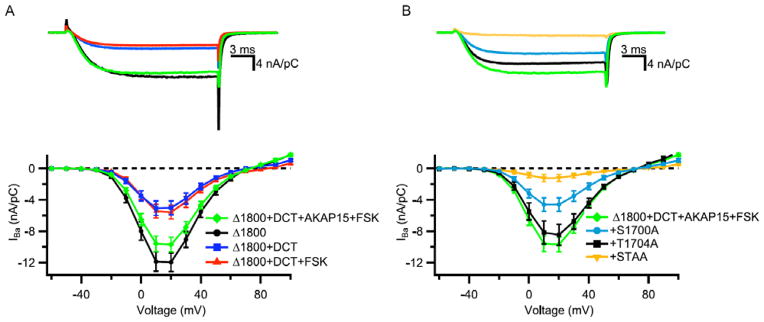
In order to test the significance of the autoinhibitory signaling complex of CaV1.2 channels in PKA regulation, we developed methods to build this signaling complex in nonmuscle cells. The tsA-201 cell line of human embryonic kidney cells expresses the transfected α1 subunit of CaV1.2 channels in full-length form, without proteolytic truncation [67]. Therefore, in the absence of this proteolytic processing reaction, the α1 subunit can be expressed as full-length, truncated, or truncated plus distal forms by expressing cDNAs encoding the full-length or truncated forms of the α1 subunit, without or with a separate cDNA encoding the distal C-terminus [69, 70]. Analysis of the PKA regulation of these three forms of CaV1.2 channels was very revealing [70]. The full-length form of the α1 subunit was not regulated by activation of PKA signaling with forskolin [70], as had already been observed by many investigators (eg., [71]). The truncated form of the α1 subunit had substantially increased activity but also was not regulated by PKA [70]. However, the autoinhibitory complex of truncated α1 subunit plus the distal C-terminal domain was up-regulated by PKA phosphorylation when it was co-expressed with a carefully titrated amount of AKAP15 [70]. Expression of CaV1.2Δ1800 alone gave large Ba2+ currents (Fig. 4A, black), which were strongly reduced by co-expression of the distal C-terminus (Fig. 4A, blue). Addition of forskolin had no effect on this autoinhibited complex (Fig. 4A, red). However, if AKAP15 was also co-expressed, substantial up-regulation of CaV1.2 channel activity was observed (Fig. 4A, green). Careful titration of AKAP15 expression was required to prevent the dominant-negative effect of the AKAP due to sequestering PKA away from the CaV1.2 channels when AKAP15 was expressed in excess. The peak CaV1.2 channel activity in this reconstituted signaling complex was increased approximately 2- to 2.5-fold compared to basal activity in unstimulated tsA-201 cells (Fig. 4; [70]). This level of regulation is comparable to that in dissociated cardiac myocytes from rodent hearts.
Fig. 4. Regulation of CaV1.2 channel activity by optimal expression of cDNA encoding CaV1.2 channel subunits and AKAP15.
(A) Representative Ba2+ currents (Top) and current-voltage relationships (Bottom) of CaV1.2 channels expressed in tsA-201 cells by co-transfection of the indicated cDNAs encoding CaV1.2Δ1800, distal C-terminus (DCT), AKAP15, and 5 μM Forskolin (FSK). (B) Representative Ba2+ currents (Top) and current-voltage relationships (Bottom) of CaV1.2 channels expressed in tsA-201 cells by co-transfection of the indicated cDNAs encoding CaV1.2Δ1800, distal C-terminus (DCT), AKAP15, and 5 μM Forskolin (FSK) with wild-type CaV1.2Δ1800 and mutants S1700A, T1704A, and S1700A/T1704A. Mean±SEM; significance determined by ANOVA.
The basal activity of CaV1.2 channels in tsA-201 cells is regulated by protein phosphorylation [70], as it is in cardiac myocytes [72]. Inhibition of protein phosphorylation with a broad-spectrum kinase inhibitor (RO 31-8220; [72]) reduced CaV1.2 channel activity to 50% of the level of basal activity of untreated cells, giving a full dynamic range of 3.6- to 4-fold for regulation of CaV1.2 channel activity by protein phosphorylation [70]. Overall, these results showed that both an autoinhibitory signaling complex of truncated α1 subunit plus distal C-terminal domain and a carefully titrated amount of AKAP were required for effective regulation by PKA and explained why it had been so difficult to observe consistent regulation of CaV1.2 channels transfected under other conditions.
Differential regulation by AKAPs
Because multiple AKAPs can support PKA regulation of CaV1.2 channels, it is of interest to compare their regulatory properties under controlled conditions in vitro. As a first step in that direction, we studied regulation of CaV1.2 channels in transfected human embryonic kidney cells by AKAP15 and AKAP79 (designated AKAP79 in human; AKAP150 in mouse) [73]. Surprisingly, AKAP79 is not effective in supporting up-regulation of CaV1.2 channels in transfected cells following activation of adenylyl cyclase by forskolin under conditions where AKAP15 is effective [73]. AKAP79 binds several signaling proteins, including protein kinase C and the calcium-regulated phosphatase calcineurin [64]. We found that mutation of AKAP79 to prevent binding of calcineurin, as described previously [64], allowed it to support up-regulation of CaV1.2 channel activity via activation of adenylyl cyclase with forskolin [73]. These results establish a new paradigm for AKAP regulation in which multiple AKAPs bind to a common site on a regulatory target and bring different signaling proteins into the complex to alter its regulation. It will be of great interest to determine whether the AKAPs associated with CaV1.2 channels at the modified leucine-zipper interaction site change with development, physiological regulation, or pathophysiological events.
SITES OF REGULATION OF CaV1.2 CHANNELS BY PROTEIN PHOSPHORYLATION
Sites of regulation of CaV1.2 channels in transfected nonmuscle cells
Successful reconstitution of the CaV1.2 signaling complex in nonmuscle cells allowed direct testing of potential sites of regulation by protein phosphorylation. The in vivo phosphorylation sites we discovered by mass spectrometry in skeletal muscle CaV1.1 channels, Ser1575 and Thr1579, are conserved in CaV1.2 channels as Ser1700 and Thr1704 [55, 70]. Moreover, Ser1700 is phosphorylated in cardiac myocytes in vivo in response to β-adrenergic regulation [31]. Mutation of these sites to Ala completely prevented regulation of CaV1.2 channels by PKA in transfected human embryonic kidney cells (Fig. 4B [70]). Mutation of Ser1700 and Thr1704 individually reduced CaV1.2 channel activity (Fig. 4B, black and green), whereas mutation of both sites nearly completely prevented up-regulation by PKA phosphorylation (Fig. 4B, yellow [70]). These results indicate that interplay between phosphorylation of Thr1704 by casein kinase II and Ser1700 by PKA controls both basal and stimulated levels of CaV1.2 channel activity in transfected nonmuscle cells.
Requirement for the distal C-terminus for regulation of CaV1.2 channels in vivo
A critical test of the functional role of the distal C-terminal in regulation of CaV1.2 channels in vivo is deletion of distal C-terminus from the CaV1.2 protein. By introducing a STOP codon at the point of proteolytic truncation of CaV1.2 in the mouse, we created a channel whose regulation by the distal C-terminal was completely prevented [74]. The resulting ΔDCT mice were surprisingly seriously impaired. All mice died perinatally from severe cardiac hypertrophy and heart failure [74]. Isolation of cardiomyocytes at P18 and maintenance in cell culture allowed studies of CaV1.2 channel channel regulation. We found that the level of CaV1.2 current was much reduced and β-adrenergic up-regulation of CaV1.2 channel activity was completely lost in myocytes from ΔDCT mice. The reduction of basal CaV1.2 current was caused by failure of expression or premature degradation, because the gating charge associated with CaV1.2 channel activation was reduced comparably to the CaV1.2 current [74]. Deletion of a smaller portion of the distal C-terminal also resulted in neonatal heart failure [75]. These dramatic phenotypes indicate that regulation of CaV1.2 channels by the distal C-terminal domain is required for normal cardiac development and function in vivo.
Requirement of Ser1700 and Thr1704 for basal and β-adrenergic regulation of CaV1.2 channels in vivo
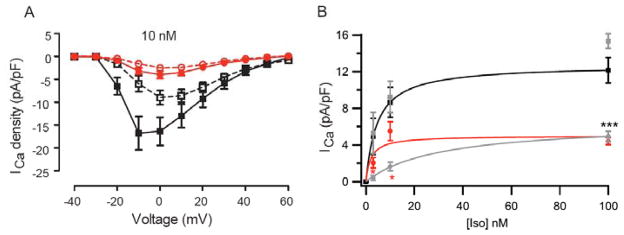
The striking effects of deletion of the distal C-terminal pointed to phosphorylation of Ser1700 and Thr1704 as crucial elements in cardiovascular homeostasis and regulation in vivo. To test this hypothesis directly, we mutated Ser1700 to Ala alone or together with Thr1704 in separate mouse lines. Ser1700Ala (SA) mice [77] and Ser1700Ala/Thr1704Ala (STAA) mice (Fig. 5A [76]) have reduced basal CaV1.2 channel activity (Fig. 5A, open symbols) and severely reduced β-adrenergic-stimulated CaV1.2 channel activity (Fig. 5A, closed symbols [76, 77]), as predicted from our studies in transfected non-muscle cells. The concentration dependence of increase in CaV1.2 channel activity by activation of β-adrenergic receptors with isoproterenol is shifted five-fold to higher concentrations by the Ser1700Ala/Thr1704Ala double mutation but not by the single Ser1700Ala mutation (Fig. 5B [77]). These results suggest that phosphorylation of Thr1704 by casein kinase II increases the sensitivity of regulation by phosphorylation of Ser1700 by PKA. This reduction in basal CaV1.2 channel activity and β-adrenergic up-regulation in these mutant mice leads to reduced β-adrenergic stimulation of cardiac myocyte contraction, though the reduction in cellular contractility is smaller than the reduction in CaV1.2 channel current, suggesting that there is a greater reserve for contractility or that compensatory mechanisms have been engaged. Both SA mice and STAA mice have substantially reduced maximal exercise capacity in a treadmill test in which they are forced to run up an incline to escape a foot shock, a test of the fight-or-flight response in vivo [76, 77]. Moreover, SA and STAA mice have substantial cardiac hypertrophy by young adulthood [76, 77], and both mouse lines suffer severe heart failure and premature deaths beginning after 200 days of age (unpublished results). The functional deficit of STAA mice is greater at the cellular level (Fig. 5B), and these mice begin to die prematurely approximately 50 days before the SA mutant mice.
Fig. 5. Reduced basal Ca2+ current and impaired response to β-adrenergic activation in adult cardiomyocytes.
(A) ICa was recorded under basal conditions and 5 min after application of 10 nM Iso in WT and STAA cardiomyocytes. Basal current was stable for two min before addition of Iso. (B) Baseline subtracted Iso-induced increment in ICa density plotted against Iso concentration. *** p<0.001, WT vs. SA. *, p<0.05 SA vs STAA. Modified from [76, 77].
Contrasting views of regulation of CaV1.2 channels
The molecular mechanism and sites of regulation of CaV1.2 channels in vivo have been investigated in other experimental preparations using pharmacologically tagged CaV1.2 channels that are insensitive to regulation by dihydropyridines (DHPs) [78, 79]. When such DHP-insensitive CaV1.2 channels are virally expressed in cardiac myocytes, and endogenous CaV1.2 channels are blocked by treatment with a DHP, normal β-adrenergic regulation is observed for virally expressed full-length CaV1.2 channels, which likely are proteolytically processed in the infected myocytes. CaV1.2 channels with the mutation Ser1928Ala and CaV1.2 channels co-expressed with CaVβ subunits having mutations in proposed phosphorylation sites also have normal β-adrenergic regulation [79]. In contrast, no regulation is observed for CaV1.2 channels truncated to remove the distal C-terminus [79]. All of these results are consistent with our experimental findings in transfected nonmuscle cells and in mutant mouse lines presented above. In contrast, in a different experimental system in which wild-type and mutant CaV1.2 channels are expressed from a transgene controlled by a Tet-on promoter system, a similar ratio of stimulation of CaV1.2 current by Iso was observed for wild-type CaV1.2 and mutants with Ser1700 and Thr1704 mutated to Ala [78]. Two key experimental differences may contribute to these apparent differences in results. First, the stoichiometry of components of the autoinhibitory signaling complex of CaV1.2 channels is crucial [70, 73], but it is not known whether correct stoichiometry of components is obtained for CaV1.2 channels expressed from transgenes. Second, in the Tet-on system, the basal level of CaV1.2 current is not known, so the stimulation ratio is calculated against a denominator whose real absolute value is unknown and may vary among conditions [78]. Indeed, it is clear from our studies of SA and STAA mice that these mutations substantially reduce basal CaV1.2 current, thereby reducing the denominator in the stimulation ratio of Iso/basal. In this situation, it is not valid to use the classical stimulation ratio as a metric of β-adrenergic stimulation because the denominator is not fixed and is reduced by the mutations. For example, in an extreme case, a mutation that only reduces basal CaV1.2 current would increase the stimulation ratio and appear to increase β-adrenergic regulation. Further experiments in which the stoichiometry of the CaV1.2 signaling complex can be established and both the basal Cav1.2 channel activity and the stimulated CaV1.2 channel activity can be measured accurately will be needed to critically assess the differences between the results with this Tet-on expression system and those presented here with mutant mouse lines.
MOLECULAR MECHANISM OF REGULATION OF CAV1 CHANNELS IN CARDIAC HOMEOSTASIS AND IN THE FIGHT-OR-FLIGHT RESPONSE
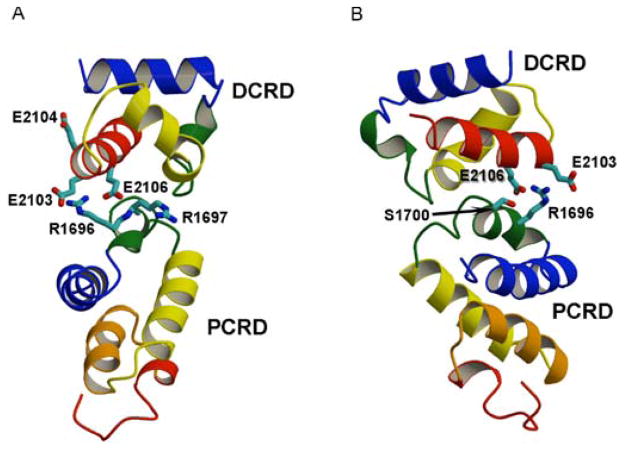
Our results point to a complex, local mode of regulation of the CaV1.1 and CaV1.2 channels (Fig. 1B). First, as part of the in vivo processing and assembly of these channel proteins in skeletal and cardiac muscle, the C-terminal domains are proteolytically cleaved at analogous positions near their center, Ala1664 or Ala1800 respectively, and the distal C-terminus remains attached to the transmembrane core of the channel through interaction with the proximal C-terminal domain [43, 44, 47, 57]. This interaction has a potent autoinhibitory influence on the activity of the CaV1.2 channel [69]. The point of regulatory interaction involves two bundles of alpha helices, which bind to each other in part through two Arg residues (Arg1696, Arg1697) in the proximal C-terminal domain forming ion pairs with a set of three negatively charged Glu residues (Glu2103, Glu2104, and Glu2106) on one face of an alpha helix in the distal C-terminal domain (Fig. 6 [69]). Neutralization of the Arg residues by mutation to Gln reduces the inhibitory interaction, whereas neutralizing both Arg residues and Glu residues prevents autoinhibitory regulation completely [69]. Exhaustive proteomic analysis revealed two sites of in-vivo phosphorylation in CaV1.1 channels (Ser1575 and Thr1579), and Ser1575 is phosphorylated by PKA in CaV1.1 channels in rabbit skeletal muscle in vivo during β-adrenergic stimulation [55]. These sites are conserved as Ser1700 and Thr1704 in CaV1.2 channels. Ser1700 is located in a PKA phosphorylation site containing Arg1696 and Arg1697 as part of its consensus sequence (Fig. 6B; [55, 70]).
Fig. 6. The docking model of the proximal and distal C-terminal domains in the CaV1.2 channel signaling complex.
(A) Shown in ribbon representation with their α-helical regions colored. Side chains of R1696 and R1697 in the PCRD are shown in stick representation with nitrogen atoms in blue. Side chains of E2103, E2104, and E2106 in the DCRD are shown in stick representation with oxygen atoms in red. (B) Rotated view of the model in panel A showing the side chain of S1700 in stick representation with the oxygen atom in red.
An A-Kinase Anchoring Protein interacts with a site in the distal C-terminal domain of CaV1.1 and CaV1.2 channels, and binding of an AKAP there is required for PKA regulation of both channel types [43, 44, 51–54, 57, 61]. Regulation of CaV1.2 channels at physiological levels can be reconstituted in nonmuscle cells by co-expression of carefully controlled amounts of truncated CaV1.2Δ1800, the distal C-terminal domain, the CaVβ and CaVα2δ subunits, and AKAP15 [70]. In this reconstituted system, phosphorylation of Ser1700 and Thr1704 regulates basal channel activity, whereas phosphorylation of Ser1700 is responsible for up-regulation of channel activity by β-adrenergic/PKA phosphorylation [70].
The key role of this regulatory system in vivo is illustrated by our studies of mutant mice. Deletion of the distal C-terminus in vivo causes reduced channel expression, loss of regulation by β-adrenergic/PKA signaling, and perinatal heart failure [74]. Mutation of both Ser1700 and Thr1704 to Ala or mutation of only Ser1700 to Ala reduces basal channel activity, without substantial reduction in channel expression, and leads to impaired contractility, reduced exercise capacity in a fight-or-flight task, and cardiac hypertrophy [76, 77]. The double mutation has greater effect at low levels of β-adrenergic stimulation, suggesting that phosphorylation of Thr1704 enhances the effectiveness of phosphorylation of Ser1700 in relieving autoinhibition and increasing channel activity.
This complex molecular model now accounts for most of the β-adrenergic stimulation of CaV1.2 channel activity, and our studies in mutant mice show that this mechanism is also required for regulation of the basal level of CaV1.2 channel activity and for normal cardiac homeostasis. However, this story remains incomplete. Future experiments should be focused on identification of additional PKA phosphorylation sites that may be required for the remaining regulation of CaV1.2 channels in STAA mice using mass spectrometry and other incisive methods and on analysis of the functional significance of those sites in vitro in reconstituted nonmuscle cells and in vivo in mutant mice. Looking back, one can see that discovery of the cardiac calcium current by Harald Reuter has led eventually to molecular understanding of this important cell signaling protein and its essential role in β-adrenergic and homeostatic regulation of the heart. In the future, it is likely that this research direction will provide new insights into heart failure and other aspects of cardiovascular disease.
Footnotes
Send Orders for Print-Reprints and e-prints to reprints@benthamscience.ae
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
References
- 1.Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- 2.Tsien RW. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. Excitation-contraction coupling in vertebrate skeletal muscle: A tale of two calcium channels. Cell. 1991;64:871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe T, Mikami A, Niidome T, Numa S, Adams BA, Beam KG. Structure and function of voltage-dependent calcium channels from muscle. Ann N Y Acad Sci. 1993;707:81–86. doi: 10.1111/j.1749-6632.1993.tb38044.x. [DOI] [PubMed] [Google Scholar]
- 7.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967;192:479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuter H. Slow inactivation of currents in cardiac Purkinje fibres. The Journal of physiology. 1968;197:233–253. doi: 10.1113/jphysiol.1968.sp008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuter H, Beeler GW., Jr Calcium current and activation of contraction in ventricular myocardial fibers. Science. 1969;163:399–401. doi: 10.1126/science.163.3865.399. [DOI] [PubMed] [Google Scholar]
- 11.Beeler GW, Jr, Reuter H. The relation between membrane potential membrane currents and activation of contraction in ventricular myocardial fibres. The Journal of physiology. 1970;207:211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beeler GW, Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. The Journal of physiology. 1970;207:191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettes LS, Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. The Journal of physiology. 1974;240:703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuter H, Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. The Journal of physiology. 1977;264:17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 16.Llinas R, Sugimori M, Hillman DE, Cherksey B. Distribution and functional significance of the P-type, voltage-dependent calcium channels in the mammalian central nervous system. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 17.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 18.Reuter H, Scholz H. The regulation of calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsien RW, Giles W, Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nature: New biology. 1972;240:181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- 20.Tsien RW. Adrenaline-like effects of intracellular iontophoresis of cyclic AMP in cardiac Purkinje fibres. Nature New Biol. 1973;245:120–122. doi: 10.1038/newbio245120a0. [DOI] [PubMed] [Google Scholar]
- 21.Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982;298:576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- 22.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 23.Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982;298:576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- 24.Brum G, Flockerzi V, Hofmann F, Osterrieder W, Trautwein W. Injection of catalytic subunit of cAMP-dependent protein kinase into isolated cardiac myocytes. Pflugers Archiv: European journal of physiology. 1983;398:147–154. doi: 10.1007/BF00581064. [DOI] [PubMed] [Google Scholar]
- 25.Kameyama M, Hofmann F, Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Archiv: European journal of physiology. 1985;405:285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- 26.Kameyama M, Hescheler J, Hofmann F, Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Archiv: European journal of physiology. 1986;407:123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- 27.Catterall WA. Structure and regulation of voltage-gated calcium channels. Annu Rev Cell Dev Bio. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 28.Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- 29.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 30.Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 31.Lundby A, Andersen MN, Steffensen AB, Horn H, Kelstrup CD, Francavilla C, Jensen LJ, Schmitt N, Thomsen MB, Olsen JV. In vivo phosphoproteomics analysis reveals the cardiac targets of beta-adrenergic receptor signaling. Science signaling. 2013;6:rs11. doi: 10.1126/scisignal.2003506. [DOI] [PubMed] [Google Scholar]
- 32.Cairns SP, Dulhunty AF. Beta-adrenergic potentiation of E-C coupling increases force in rat skeletal muscle. Muscle & nerve. 1993;16:1317–1325. doi: 10.1002/mus.880161208. [DOI] [PubMed] [Google Scholar]
- 33.Cairns SP, Dulhunty AF. The effects of beta-adrenoceptor activation on contraction in isolated fast- and slow-twitch skeletal muscle fibres of the rat. British journal of pharmacology. 1993;110:1133–1141. doi: 10.1111/j.1476-5381.1993.tb13932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oz M, Frank GB. Decrease in the size of tetanic responses produced by nitrendipine or by extracellular calcium ion removal without blocking twitches or action potentials in skeletal muscle. The Journal of pharmacology and experimental therapeutics. 1991;257:575–581. [PubMed] [Google Scholar]
- 35.Kernell D, Eerbeek O, Verhey BA. Relation between isometric force and stimulus rate in cat’s hindlimb motor units of different twitch contraction time. Experimental brain research. 1983;50:220–227. doi: 10.1007/BF00239186. [DOI] [PubMed] [Google Scholar]
- 36.Fitts RH, McDonald KS, Schluter JM. The determinants of skeletal muscle force and power: their adaptability with changes in activity pattern. Journal of biomechanics. 1991;24(Suppl 1):111–122. doi: 10.1016/0021-9290(91)90382-w. [DOI] [PubMed] [Google Scholar]
- 37.Arreola J, Calvo J, Garcia MC, Sanchez JA. Modulation of calcium channels of twitch skeletal muscle fibres of the frog by adrenaline and cyclic adenosine monophosphate. The Journal of physiology. 1987;393:307–330. doi: 10.1113/jphysiol.1987.sp016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis BM, Catterall WA. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984;23:2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- 39.Flockerzi V, Oeken HJ, Hofmann F. Purification of a functional receptor for calcium channel blockers from rabbit skeletal muscle microsomes. Eur J Biochem. 1986;161:217–224. doi: 10.1111/j.1432-1033.1986.tb10145.x. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis BM, Catterall WA. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985;82:2528-25-32. doi: 10.1073/pnas.82.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosey MM, Barhanin J, Schmid A, Vandaele S, Ptasienski J, O’Callahan C, Cooper C, Lazdunski M. Photoaffinity labelling and phosphorylation of a 165 kilodalton peptide associated with dihydropyridine and phenylalkylamine-sensitive calcium channels. Biochemical and biophysical research communications. 1987;147:1137–1145. doi: 10.1016/s0006-291x(87)80188-2. [DOI] [PubMed] [Google Scholar]
- 43.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc Natl Acad Sci U S A. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the α1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci U S A. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rotman EI, De Jongh KS, Florio V, Lai Y, Catterall WA. Specific phosphorylation of a COOH-terminal site on the full-length form of the α1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase. J Biol Chem. 1992;267:16100–16105. [PubMed] [Google Scholar]
- 46.Rotman EI, Murphy BJ, Catterall WA. Sites of selective cAMP-dependent phosphorylation of the L-type calcium channel α1 subunit from intact rabbit skeletal muscle myotubes. J Biol Chem. 1995;270:16371–16377. doi: 10.1074/jbc.270.27.16371. [DOI] [PubMed] [Google Scholar]
- 47.Hulme JT, Konoki K, Lin TW, Gritsenko MA, Camp DG, 2nd, Bigelow DJ, Catterall WA. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1.1 channels in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5274–5279. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 49.Johnson BD, Brousal JP, Peterson BZ, Gallombardo PA, Hockerman GH, Lai Y, Scheuer T, Catterall WA. Modulation of the cloned skeletal muscle L-type Ca2+ channel by anchored cAMP-dependent protein kinase. J Neurosci. 1997;17:1243–1255. doi: 10.1523/JNEUROSCI.17-04-01243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson BD, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 52.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, 3rd, Scheuer T, Catterall WA, Murphy BJ. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 53.Fraser IDC, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 55.Emrick MA, Sadilek M, Konoki K, Catterall WA. Beta-adrenergic-regulated phosphorylation of the skeletal muscle Ca(V)1.1 channel in the fight-or-flight response. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18712–18717. doi: 10.1073/pnas.1012384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel α1 subunit. J Biol Chem. 1993;268:19451–19457. [PubMed] [Google Scholar]
- 57.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by cAMP-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 58.Puri TS, Gerhardstein BL, Zhao XL, Ladner MB, Hosey MM. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry. 1997;36:9605–9615. doi: 10.1021/bi970500d. [DOI] [PubMed] [Google Scholar]
- 59.Haase H, Bartel S, Karczewski P, Morano I, Krause EG. In-vivo phosphorylation of the cardiac L-type calcium channel beta-subunit in response to catecholamines. Mol Cell Biochem. 1996;163–164:99–106. doi: 10.1007/BF00408645. [DOI] [PubMed] [Google Scholar]
- 60.Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J. Identification of PK-A phosphorylation sites in the carboxyl terminus of L-type calcium channel α1 subunits. Biochemistry. 1996;35:9400–9406. doi: 10.1021/bi960683o. [DOI] [PubMed] [Google Scholar]
- 61.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones BW, Brunet S, Gilbert ML, Nichols CB, Su T, Westenbroek RE, Scott JD, Catterall WA, McKnight GS. Cardiomyocytes from AKAP7 knockout mice respond normally to adrenergic stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17099–17104. doi: 10.1073/pnas.1215219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 64.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Eick RT, Hosey MM. C-terminal fragments of the α1C (Cav1. 2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated α1C subunits. J Biol Chem. 2001;276:21089–21097. doi: 10.1074/jbc.M008000200. [DOI] [PubMed] [Google Scholar]
- 66.Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C terminus of the alpha(1C) subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- 67.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei X, Neely A, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J. Biol. Chem. 1994;269:1635–1640. [PubMed] [Google Scholar]
- 69.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. The Journal of physiology. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Science signaling. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zong X, Schreieck J, Mehrke G, Welling A, Schuster A, Bosse E, Flockerzi V, Hofmann F. On the regulation of the expressed L-type calcium channel by cAMP-dependent phosphorylation. Pflugers Arch. 1995;430:340–347. doi: 10.1007/BF00373908. [DOI] [PubMed] [Google Scholar]
- 72.duBell WH, Rogers TB. Protein phosphatase 1 and an opposing protein kinase regulate steady-state L-type Ca2+ current in mouse cardiac myocytes. The Journal of physiology. 2004;556:79–93. doi: 10.1113/jphysiol.2003.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuller MD, Fu Y, Scheuer T, Catterall WA. Differential regulation of CaV1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. The Journal of general physiology. 2014;143:315–324. doi: 10.1085/jgp.201311075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu Y, Westenbroek RE, Yu FH, Clark JP, 3rd, Marshall MR, Scheuer T, Catterall WA. Deletion of the distal C terminus of CaV1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J Biol Chem. 2011;286:12617–12626. doi: 10.1074/jbc.M110.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Domes K, Ding J, Lemke T, Blaich A, Wegener JW, Brandmayr J, Moosmang S, Hofmann F. Truncation of murine CaV1. 2 at Asp-1904 results in heart failure after birth. J Biol Chem. 2011;286:33863–33871. doi: 10.1074/jbc.M111.252312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19621–19626. doi: 10.1073/pnas.1319421110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Basal and beta-adrenergic regulation of the cardiac calcium channel CaV1. 2 requires phosphorylation of serine 1700. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16598–16603. doi: 10.1073/pnas.1419129111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang L, Katchman A, Samad T, Morrow JP, Weinberg RL, Marx SO. Beta-adrenergic regulation of the L-type Ca2+ channel does not require phosphorylation of alpha1C Ser1700. Circulation research. 2013;113:871–880. doi: 10.1161/CIRCRESAHA.113.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circulation research. 2006;98:e11–18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Catterall WA. Signaling complexes of voltage-gated sodium and calcium channels. Neuroscience letters. 2010;486:107–116. doi: 10.1016/j.neulet.2010.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]