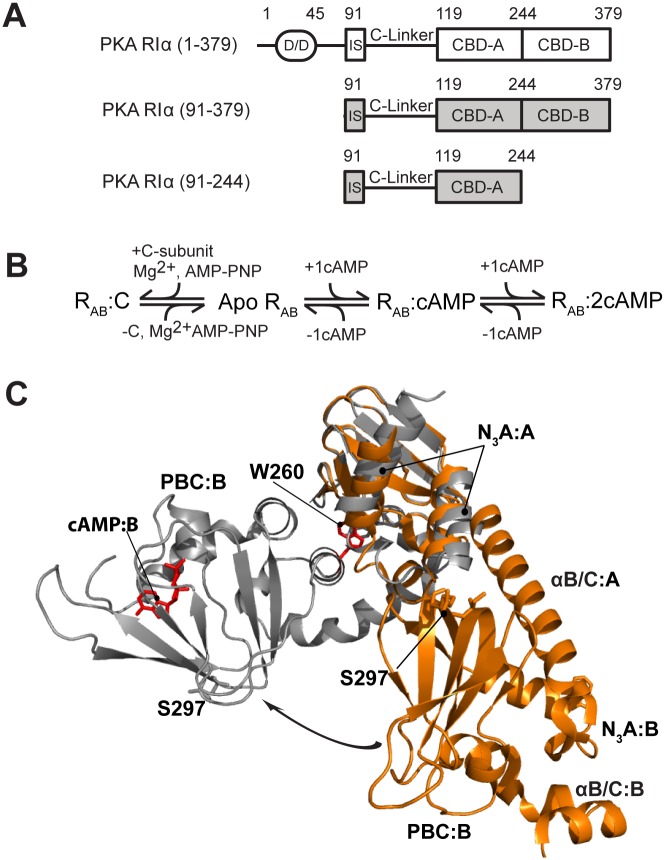
Fig 1. Construct design and architecture of PKA RIα.
(A) Domain organization of the RIα subunit of PKA and its functional constructs that ensure cAMP-dependent inhibition of the catalytic subunit (C). (B) Binding equilibria of PKA RIα. Even when apo RIα is a transient low-population intermediate, it is still a critical thermodynamic determinant of the cAMP-dependent regulation of PKA. (C) Structures of PKA RIα (91–379) bound to either cAMP (grey, PDB code 1RGS) or the C-subunit (orange, PDB code 2QCS for the R333K mutant of RIα (91–379)). The two structures are superimposed through the β-barrel of cAMP-binding domain (CBD) A. Selected regions and residues are labeled. The arrow shows the change in the position of CBD-B relative to CBD-A occurring upon cAMP-binding.