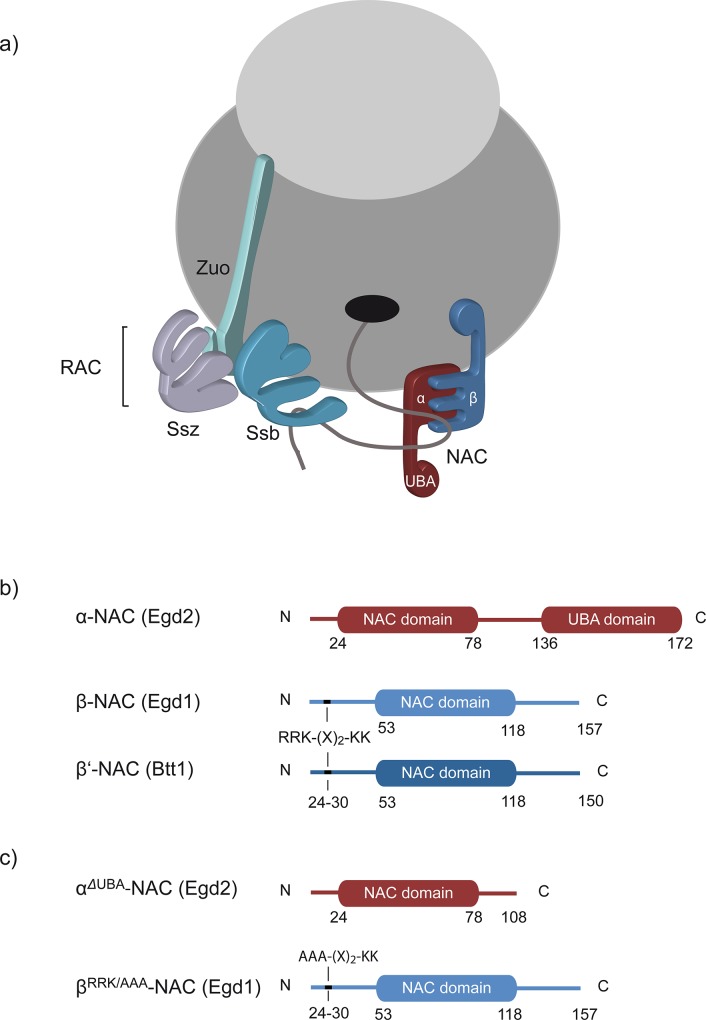
Fig 1. Ribosome-associated chaperones from S. cerevisiae.
a) The Hsp70/Hsp40-chaperone system that consists of RAC (Ssz and Zuo), shown in purple and light green, and Ssb, shown in light blue, forms a functional triad at the ribosome. In addition, β-NAC (shown in blue) and α-NAC (shown in red) that contains a C-terminal UBA (ubiquitin-associated) domain constitute the stable heterodimeric αβ-NAC complex which binds to the ribosome via the ribosome-binding motif in the β-subunit. Both, NAC and Ssb can interact directly with the nascent chain. b) Schematic representation of the different NAC subunits. α-NAC (shown in red) contains a NAC domain and a UBA domain. Besides the NAC domain the two different β-subunits (shown in light and dark blue) also contain a conserved ribosome-binding motif present in their N-termini. c) Schematic drawing of the two NAC mutants investigated in this study. αΔUBA-NAC (shown in red) lacks the C-terminal UBA domain and part of the linker region. In βRRK/AAA-NAC (shown in blue) the conserved RRK-(X)2-KK motif was mutated to AAA-(X)2-KK to abolish ribosome binding.