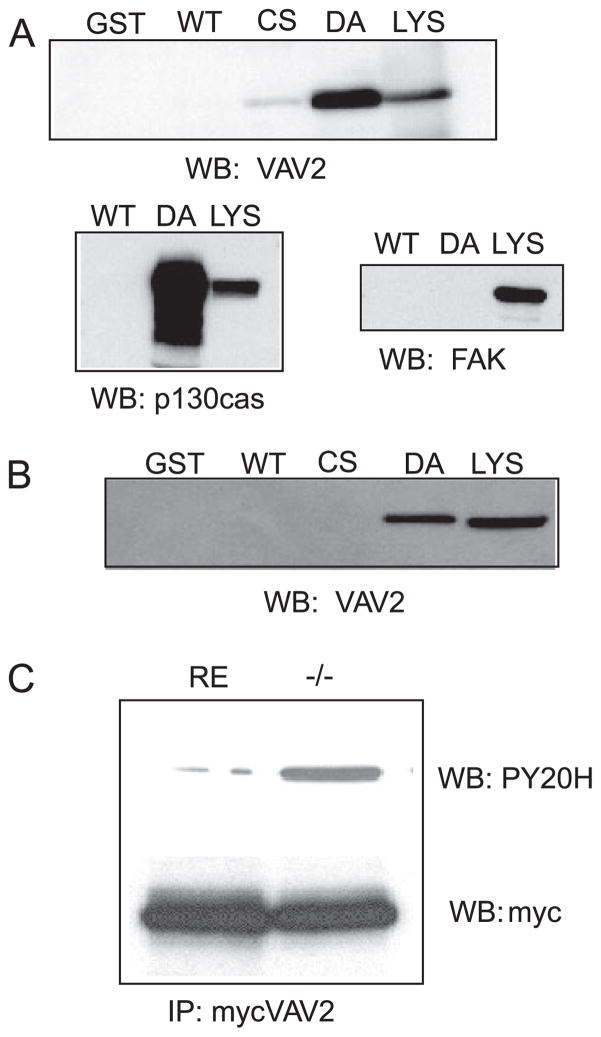
FIGURE 4. VAV2 is a substrate of PTP-PEST.
A, to determine whetherVAV2 was a direct target of PTP-PEST, a substrate trapping technique was employed. A GST fusion protein containing the catalytic domain of PTP-PEST mutated in its active site was used as an affinity matrix to “trap” tyrosine-phosphorylated substrates. 293T cells were treated with pervanadate and then lysed. Trapping mutants or wild type (WT) PTP-PEST was used to pull down VAV2. The pull-downs were blotted (WB) with an anti-serum raised against human VAV2. Two different trapping mutants were used, PTP-PEST-CS (Cys-231 → Ser (CS)) and PTP-PEST-DA (Asp-199 → Ala (DA)), to trap VAV2. LYS, lysate. As shown in the upper blot, VAV2 is present in pull-downs using the trapping mutant but not GST alone or wild type PTP-PEST. Below is a positive control for p130CAS, a known PTP-PEST substrate, and a negative control for focal adhesion kinase, which is not a substrate. FAK, focal adhesion kinase. B, substrate trapping of endogenous VAV2 from PTP-PEST −/− fibroblasts. C, tyrosine phosphorylation of VAV2 is enhanced in PTP-PEST −/− cells. IP, immunoprecipitation.