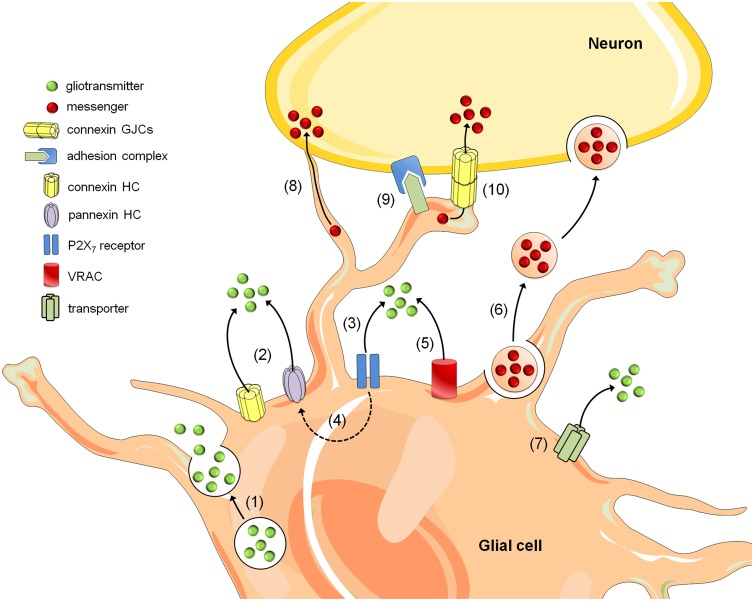
Figure 1.
Mechanisms of glia-to-neuron communication. Glial cells release gliotransmitters (e.g., glutamate, D-serine, and ATP) through Ca2+- and SNARE-dependent exocytosis (1) in addition to the release that occurs through alternative non-exocytotic pathways (see below). Depolarization, reductions in extracellular divalent cation concentrations, increases in intracellular Ca2+ and posttranslational modifications might result in the opening of connexin and pannexin hemichannels (HCs) and thus allow the release of gliotransmitters (2). Long-lasting activation of P2X7 by ATP might lead to the appearance of large currents and the rapid exchange of large molecules, including the release of gliotransmitters (3). One theory states that P2X7 receptor conductance dilates over the time and thereby allows the passage of large molecules; however, another hypothesis states that ATP activate a second non-selective permeabilization pathway (Baroja-Mazo et al., 2013). Recently, it was shown that Panx1 hemichannels might mediate this permeability for large molecules in astrocytes (4) (Iglesias et al., 2009). Additionally, gliotransmitter release may occur through volume-regulated anion channels (VRAC) (5) and different carriers and/or co-transporters acting normally or in reverse (6) (e.g., excitatory amino-acid transporters, the cystine-glutamate antiporter, and the D-serine/chloride co-transporter). Within the last decade, a growing body of evidence has indicated that glial cells can also communicate with neurons via the release of vesicles (e.g., exosomes, microparticles, and apoptotic bodies), containing different cellular messengers (e.g., mRNA, viruses, and organelles) (7). Adjacent glial cells and neurons can communicate directly through F-actin-based transient tubular connections known as tunneling nanotubes (8), via cell-to-cell contacts between membrane-bound ligand molecules and their receptors (9) or aggregates of intercellular channels known as gap junctions, which allow the exchange of small molecules (10).