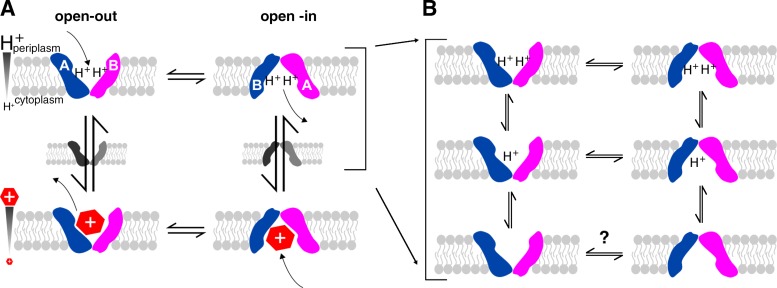
Figure 1.
The mechanism of EmrE transport. EmrE is a homodimer composed of two subunits (blue and pink) oriented antiparallel to each other and in unique conformations (distinct shapes, labeled A and B). The subunits exchange conformations to switch between open-in and open-out forms. (A) In the standard single-site alternating access model, a single active site located between the two subunits alternates between binding protons or drug. Coupling between protons and drug is achieved by preventing exchange in the apo state and only allowing one substrate to bind at a time. (B) Drug-free EmrE has more states than represented in the basic model shown in A and most, if not all, of these states exchange.