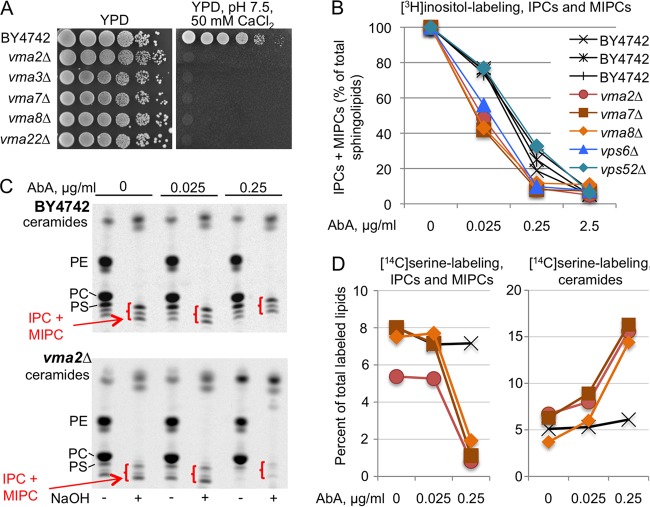
FIG 6.
AbA causes stronger inhibition of Aur1 in vma cells than in WT cells. (A) Tenfold dilutions of the indicated strains were plated on YPD or YPD with 50 mM HEPES (pH 7.5) and 50 mM CaCl2. (B) Cells growing for 14 h in inositol-free SC medium were labeled with [3H]inositol for 2 h in the presence of various concentrations of AbA. Lipid extracts were deacylated by mild base treatment and run on TLC. The amounts of mild-base-resistant sphingolipids made in the absence of AbA were set as 100% for each strain, and the amounts of sphingolipids made in the presence of AbA were calculated as a percentage thereof. (NaOH-resistant lipids accounted for 50.6% ± 3.3% and 35.5% ± 0.8% of total lipids in the WT and vma mutants, respectively.) (C) Autoradiograms of TLC plates resolving lipid extracts from WT or vma2Δ cells having been labeled with [14C]serine with or without AbA and deacylated or not with NaOH. In all TLC lanes, material from equivalent numbers of WT and mutant cells was separated. (D) Quantifications of the data of panel C (vma2Δ) plus those from vma7Δ and vma8Δ mutants labeled in the same experiment. For each of the 12 labelings (4 strains labeled under three conditions [0, 0.025, and 0.25 μg/ml of AbA]), the total amounts of label in the nondeacylated lipid extract were set as 100%, and the counts in IPCs plus MIPCs or in the stronger of the two bands in the ceramide region present after NaOH treatment were expressed as a percentage thereof. Strain symbols are as in panel B.