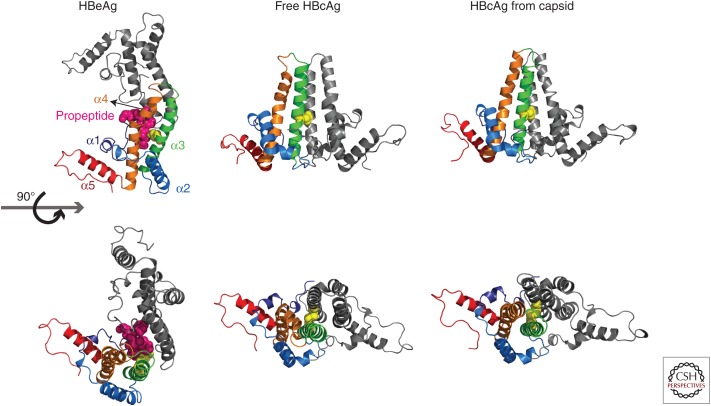
Figure 3.
Structures of hepatitis B core antigen (HBcAg) and hepatitis B e antigen (HBeAg) dimers. HBeAg harbors a 10-amino-acid-long amino-terminal extension (propeptide). The structure of the monomer structure shows modest conformational changes between free HBcAg, HBcAg in capsid, and HBeAg. The largest structural changes are found in helix α3 and α4, located at the intradimer interface (orange) (adapted from Zlotnick et al. 2013). Cysteine-7, located on the amino-terminal extension of HBeAg (3V6Z) (magenta spheres) can form a disulfide with cysteine 61 (yellow spheres). To accommodate this peptide, the monomers are rotated ∼140° about the intradimer interface from their orientation in HBcAg. In HBcAg, the monomers are parallel, and an intradimer Cys61-Cys61 disulfide can form. Structural differences in the spike region as well as the dimer–dimer interface can be observed between free HBcAg dimer (3KXS) and HBcAg from capsid (1QGT) (assembly-inactive and assembled states, respectively).