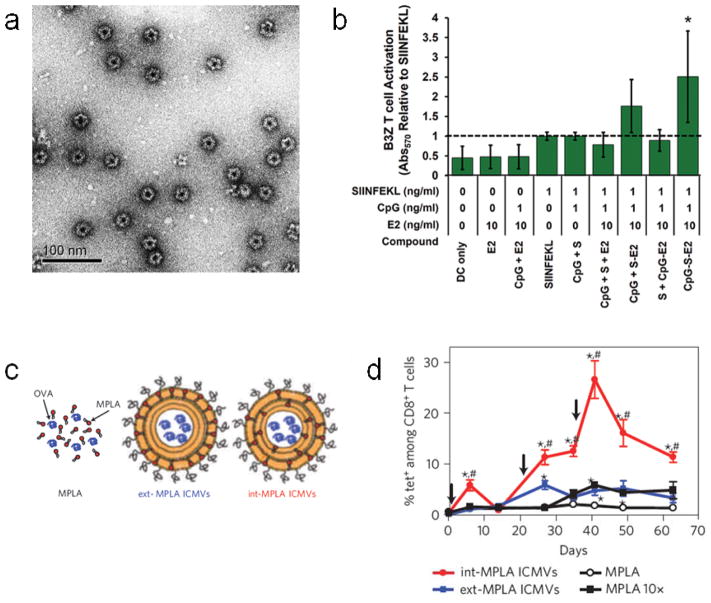
Figure 4.
Nanoparticulate subunit vaccines. (a) TEM image of negatively-stained protein-based vaccines showing virus mimicking dodecahedral geometry. (b) Activation of B3Z antigen-specific T cell hybridoma cell line by BMDCs either unloaded (DC only), or loaded with E2 vehicle (E2), soluble CpG with E2 vehicle (CpG + E2), soluble peptide antigen (SIINFEKL), soluble CpG with soluble peptide antigen (CpG + S), soluble CpG with soluble peptide antigen and E2 vehicle (CpG + S + E2), soluble CpG with peptide antigen-conjugated E2 (CpG + S-E2), soluble peptide antigen with CpG-conjugated E2 (S + CpG-E2), or peptide antigen and CpG co-loaded E2 (CpG-S-E2). (c) Diagram showing OVA-loaded ICMV vaccine formulations containing MPLA only in the outer vesicle layer (ext-MPLA ICMVs) or containing MPLA throughout the multilayers (int-MPLA ICMVs). (d) Time-dependent quantification of antigen-specific CTLs in peripheral blood after subcutaneous vaccination of naive mice with soluble MPLA in combination with soluble OVA (MPLA), ICMVs loaded with the same amount of OVA and MPLA with the MPLA either in the outer vesicle layer only (ext-MPLA ICMVs) or distributed throughout the multilayers (int-MPLA ICMVs), or with soluble OVA in combination with a 10-fold higher dose of soluble MPLA. Black arrows represent vaccination timepoints. a, b from [78], c, d from [111].