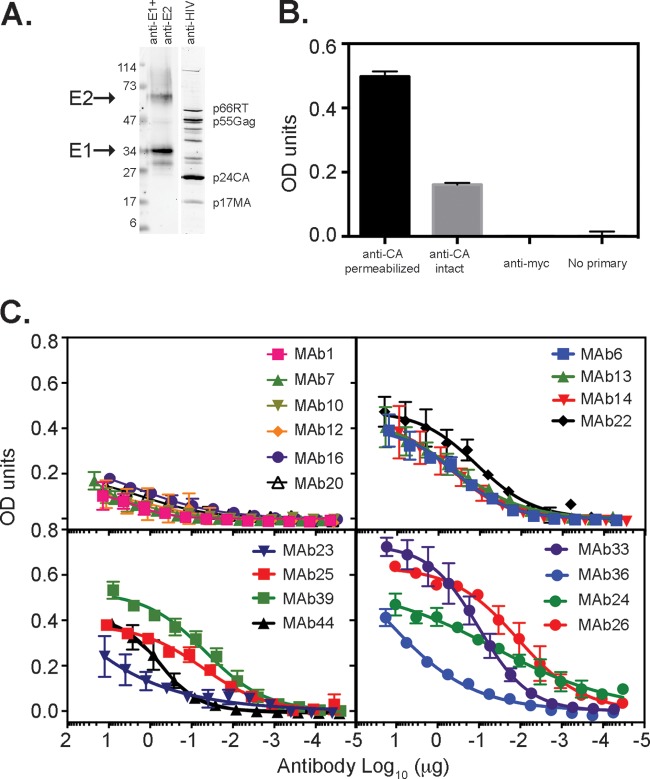
FIG 5.
Ability of MAbs to bind their epitopes on the surface of VLPs. (A) VLPs containing genotype 1a H77c E1E2 glycoproteins were pelleted through a sucrose cushion, subjected to reducing SDS-PAGE, and transferred onto nitrocellulose. Membranes were probed with a mixture of H52 (anti-E2) and A4 (anti-E1) or with IgG obtained from an HIV-positive individual. (B) Binding of anticapsid antibody to VLPs is enhanced by permeabilization with Triton X-100. Capsid protein (anti-CA) was detected with MAb183. No binding was observed by using an irrelevant MAb to a Myc epitope tag (anti-myc) or in the absence of primary antibody (No primary). (C) Ability of MAbs to bind VLPs in a direct binding ELISA. Data shown are the means ± standard deviations of data from two independent experiments. OD, optical density.