Abstract
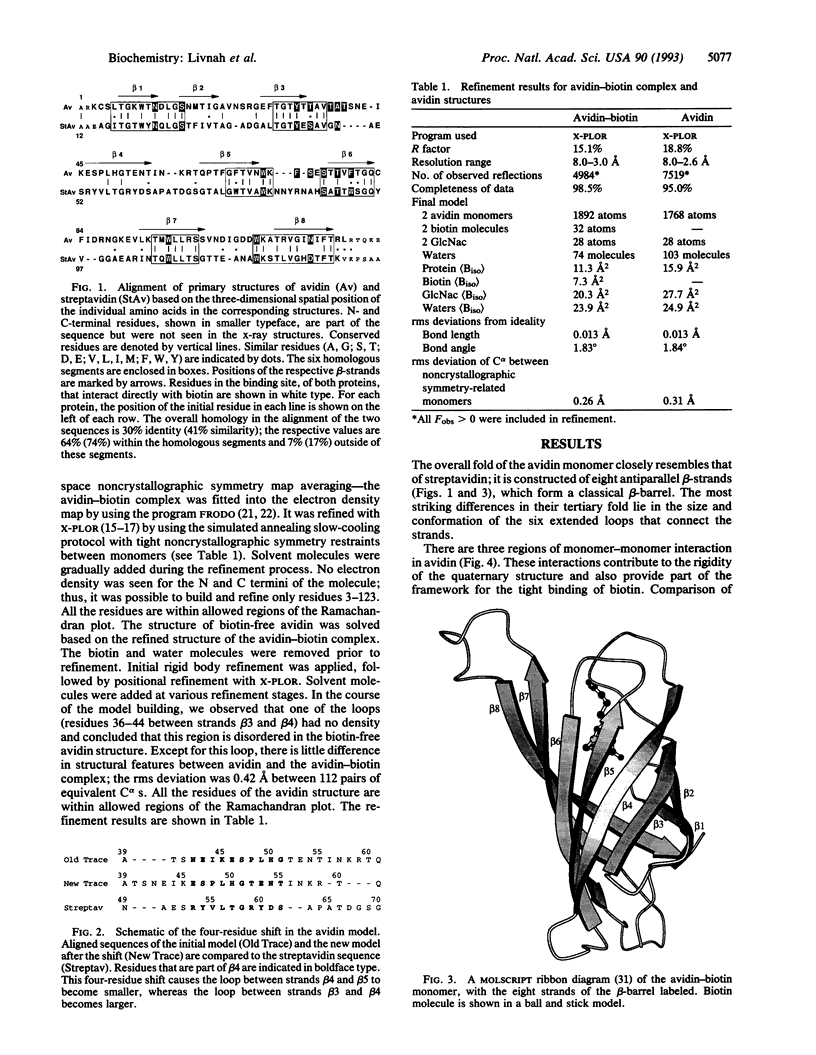
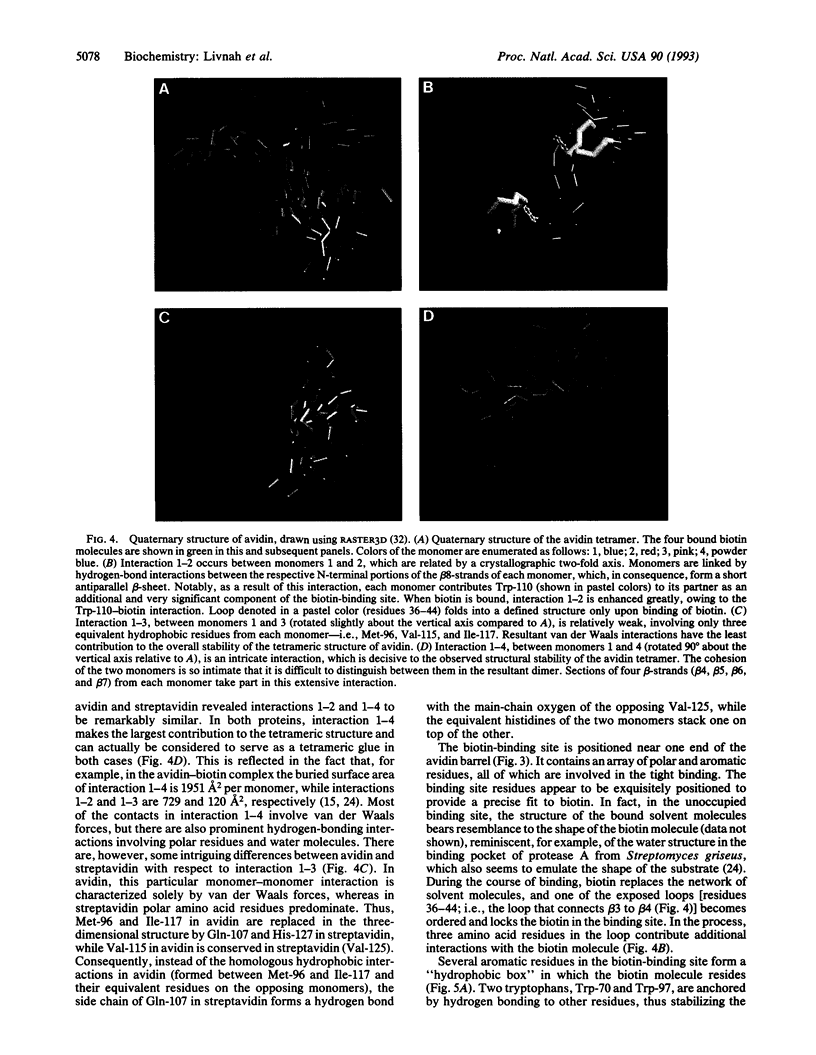
The crystal structures of a deglycosylated form of the egg-white glycoprotein avidin and of its complex with biotin have been determined to 2.6 and 3.0 A, respectively. The structures reveal the amino acid residues critical for stabilization of the tetrameric assembly and for the exceptionally tight binding of biotin. Each monomer is an eight-stranded antiparallel beta-barrel, remarkably similar to that of the genetically distinct bacterial analog streptavidin. As in streptavidin, binding of biotin involves a highly stabilized network of polar and hydrophobic interactions. There are, however, some differences. The presence of additional hydrophobic and hydrophilic groups in the binding site of avidin (which are missing in streptavidin) may account for its higher affinity constant. Two amino acid substitutions are proposed to be responsible for its susceptibility to denaturation relative to streptavidin. Unexpectedly, a residual N-acetylglucosamine moiety was detected in the deglycosylated avidin monomer by difference Fourier synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argaraña C. E., Kuntz I. D., Birken S., Axel R., Cantor C. R. Molecular cloning and nucleotide sequence of the streptavidin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1871–1882. doi: 10.1093/nar/14.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. Application of avidin-biotin technology to affinity-based separations. J Chromatogr. 1990 Jun 27;510:3–11. doi: 10.1016/s0021-9673(01)93733-1. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Krukowski A., Erickson J. W. Slow-cooling protocols for crystallographic refinement by simulated annealing. Acta Crystallogr A. 1990 Jul 1;46(Pt 7):585–593. doi: 10.1107/s0108767390002355. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Gatti G., Bolognesi M., Coda A., Chiolerio F., Filippini E., Malcovati M. Crystallization of hen egg-white avidin in a tetragonal form. J Mol Biol. 1984 Sep 25;178(3):787–789. doi: 10.1016/0022-2836(84)90253-5. [DOI] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of avidin. Tryptophan residues involved in the active site. Biochem J. 1988 Feb 15;250(1):291–294. doi: 10.1042/bj2500291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of streptavidin. Tryptophan residues involved in the active site. Biochem J. 1988 Nov 15;256(1):279–282. doi: 10.1042/bj2560279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding sites of avidin and streptavidin. Tyrosine residues are involved in the binding site. Biochem J. 1990 Jul 15;269(2):527–530. doi: 10.1042/bj2690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin and streptavidin. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Green N. M., Joynson M. A. A preliminary crystallographic investigation of avidin. Biochem J. 1970 Jun;118(1):71–72. doi: 10.1042/bj1180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller Y., Bayer E. A., Wilchek M. Studies on the biotin-binding site of avidin. Minimized fragments that bind biotin. Biochem J. 1991 Sep 1;278(Pt 2):573–585. doi: 10.1042/bj2780573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller Y., Gershoni J. M., Bayer E. A., Wilchek M. Biotin binding to avidin. Oligosaccharide side chain not required for ligand association. Biochem J. 1987 Nov 15;248(1):167–171. doi: 10.1042/bj2480167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Pinn E., Pähler A., Saenger W., Petsko G. A., Green N. M. Crystallization and preliminary X-ray investigation of avidin. Eur J Biochem. 1982 Apr;123(3):545–546. doi: 10.1111/j.1432-1033.1982.tb06566.x. [DOI] [PubMed] [Google Scholar]
- Piontek K., Chakrabarti P., Schär H. P., Rossmann M. G., Zuber H. Structure determination and refinement of Bacillus stearothermophilus lactate dehydrogenase. Proteins. 1990;7(1):74–92. doi: 10.1002/prot.340070108. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G., Weaver L. H., Wilson E., Karlsson R., Jansonius J. N. Diffraction methods for biological macromolecules. Seed enlargement and repeated seeding. Methods Enzymol. 1985;114:132–135. doi: 10.1016/0076-6879(85)14011-5. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Ohlendorf D. H., Wendoloski J. J., Salemme F. R. Structural origins of high-affinity biotin binding to streptavidin. Science. 1989 Jan 6;243(4887):85–88. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. Avidin-biotin technology ten years on: has it lived up to its expectations? Trends Biochem Sci. 1989 Oct;14(10):408–412. doi: 10.1016/0968-0004(89)90289-2. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]
- Wynne D., Wilchek M., Novogrodsky A. A chemical approach for the localization of membrane sites involved in lymphocyte activation. Biochem Biophys Res Commun. 1976 Feb 9;68(3):730–739. doi: 10.1016/0006-291x(76)91206-7. [DOI] [PubMed] [Google Scholar]