Abstract
Unlike other cyclins that positively regulate the cell cycle, cyclin G2 (CCNG2) regulates cell proliferation as a tumor suppressor gene. A decreased CCNG2 expression serves as a marker for poor prognosis in several types of cancer. The aim of the present study was to clarify the correlation of CCNG2 expression with overall survival and histopathological factors in pancreatic cancer patients. This retrospective analysis included data from 36 consecutive patients who underwent complete surgical resection for pancreatic cancer and did not undergo any preoperative therapies. The association between prognoses and the expression of CCNG2 was assessed using immunohistochemical staining. Multivariate analysis identified that the expression of CCNG2 is an independent prognostic factor. In addition, the Kaplan-Meier curve for overall survival revealed that decreased expression of CCNG2 was a consistent indicator of poor prognosis in pancreatic cancer patients (P=0.0198). A decreased CCNG2 expression significantly correlated with venous invasion in tumor specimens and the tumor invasion depth. In conclusion, CCNG2 expression inversely reflected cancer progression and may be a novel, independent prognostic marker in pancreatic cancer.
Keywords: immunohistochemical staining, cyclin G2, pancreatic cancer, overall survival
Introduction
Pancreatic cancer has the worst prognosis of all major malignancies, with a 5-year survival rate of 6% (1). At present, surgical resection is the only effective treatment in these patients; however, the 5-year survival rate following surgical resection is only 5.5–21% (2,3). Gemcitabine (GEM)-based chemotherapy is the core of multimodal therapy for pancreatic cancer and has improved patient prognosis (3). Multimodal therapies that include both chemotherapy and radiation therapy have been previously investigated and are able to reportedly improve the clinical outcome in pancreatic cancer patients (4–6). This provides several therapeutic pathways to help reduce the high refractoriness of pancreatic cancer. Therefore, identifying specific predictive markers in order to determine which patients present a poor prognosis is essential.
Cyclin G2 (CCNG2; encoded by CCNG2 gene) belongs to a family of cyclins homologous to CCNG1 (7). Cyclins positively regulate cell proliferation to a large extent. However, unexpectedly, CCNG2 has been reported to regulate cell proliferation as a tumor suppressor gene and its decreased expression is associated with malignant phenotypes in several types of cancer (8–17). Previous studies have reported that CCNG2 is involved in a variety of functions associated with cancer progression, including the regulation of cell proliferation (8–10), chemoresistance (8), DNA repair (11) and cell differentiation (12). Furthermore, our previous study demonstrated that CCNG2 was associated with chemoresistance and cancer stemness via cell apoptosis in pancreatic cancer (13). Recently, CCNG2 has been reported as a novel prognostic marker in several types of cancer (14–17). However, to date, no study has clarified the association between CCNG2 and the prognosis of pancreatic cancer. Therefore, in the present study, the association between CCNG2 expression and the clinical outcomes of 36 patients with pancreatic cancer was evaluated.
Materials and methods
Primary tumor samples
Between March 2007 and October 2012, 92 patients underwent surgery for pancreatic cancer at the Department of Gastroenterological Surgery in Osaka University (Osaka, Japan). Among these patients, 36 consecutive patients who underwent curative resection (R0) with histologically clear margins and no preoperative therapy were enrolled in the present study. All the patients were staged prior to and following surgery, according to the criteria of the Union for International Cancer Control (18). The median follow-up period was 26.4 months (range, 3.8–79.7 months), the 5-year survival rate was 29.0%, and recurrence of the disease was observed in 19 patients. GEM was administered in 21 patients as adjuvant chemotherapy (1,000 mg/m2, 3 times/month for 6 months). No radiation therapy was administered during the follow-up period. Table I summarizes the characteristics of the 36 patients. The use of resected samples was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Osaka University (approval number, 08226). Written informed consent was obtained from all the patients included in the study.
Table I.
Clinicopathological characteristics of the 36 pancreatic cancer patients.
| Parameter | Value |
|---|---|
| Mean age, years | 68.5±9.4 |
| Gender (male/female), n | 21/15 |
| Location (Ph/Pb/Pt), n | 10/21/5 |
| Lymphatic invasion (+/-), n | 26/10 |
| Venous invasion (+/-), n | 15/21 |
| Intrapancreatic perineural invasion (+/-), n | 29/7 |
| Maximal diameter, mm | 25.9±14.6 |
| Histology (well/mod/poor), n | 2/30/4 |
| pT (T1/T2/T3/T4), n | 4/4/28/0 |
| pN (+/-), n | 16/20 |
| pStage (IA/IB/IIA/IIB/III/IV), n | 4/4/12/16/0/0 |
| CCNG2 expression (+/-), n | 17/19 |
| Adjuvant therapy (+/-), n | 21/15 |
| Recurrence (+/-), n | 19/17 |
Ph/Pb/Pt, pancreatic head/body/tail; (+/-), yes/no or positive/negative; well/mod/poor, well/moderately/poorly-differentiated; pT, tumor invasion depth; pN, lymph node metastasis; CCNG2, cyclin G2.
Immunohistochemical staining
Immunohistochemical staining was performed using the method described previously (19), in order to detect CCNG2 expression in the 36 pancreatic cancer samples. Noncancerous pancreatic tissues obtained from the 36 patients were used as positive controls. Briefly, formalin-fixed, paraffin-embedded 4-µm sections were deparaffinized in xylene. Next, antigen-retrieval was performed with heat-induced epitope retrieval, at 95°C for 40 min and then the samples were incubated in methanol containing 0.3% hydrogen peroxide to block endogenous peroxidase. Following incubation with normal protein block serum (Vectastain Elite ABC kit; Vector Laboratories, Inc., Burlingame, CA, USA), the sections were incubated overnight at 4°C with an anti-CCNG2 antibody as the primary antibody (polyclonal rabbit anti-human CCNG2 antibody; 1.0 µg/ml; MBL International Corporation, Nagoya, Japan) (11). Thereafter, the staining was revealed with avidin-biotin complex reagents (Vector Laboratories Inc., Burlingame, CA, USA) using an Olympus BX50 microscope (Olympus Corporation, Tokyo, Japan) and 3,3′-diaminobenzidine. All sections were counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO, USA). Positive staining for CCNG2 was defined as detectable nuclear staining in >50% of the cancer cells.
Statistical analysis
Data are expressed as the mean ± standard deviation. The clinicopathological parameters were compared using Fisher's exact test, and the continuous variables were compared using the Mann-Whitney U test. The survival curves were plotted using the Kaplan-Meier method, while the differences between survival curves were compared using the log-rank test. P<0.05 denotes the presence of a statistically significant difference. Statistical analysis was performed using JMP software, version 10.0.2 (SAS Institute Inc., Cary, NC, USA).
Results
CCNG2 expression in pancreatic cancer tissue samples
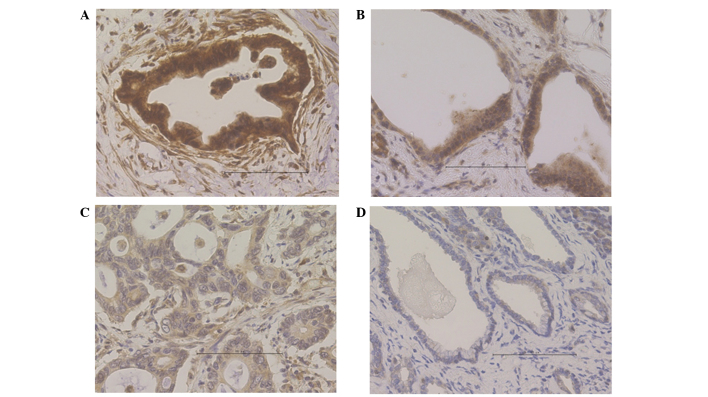
Immunohistochemical staining was performed to detect CCNG2 expression in the 36 samples included in the present study. The nuclei of normal pancreatic ductal cells were partially stained; by contrast, acinar cells, which were used as positive controls, were stained strongly in the cytoplasm and nuclei (Fig. 1). In the cancerous sections, the functional CCNG2 protein expression appeared to localize in the nucleus, although CCNG2 expression has been previously demonstrated to appear in the cytoplasm as well (12). Cases were defined as CCNG2-positive when the cells presented diffused or spotted nuclear patterns (>50% of cancer cells; Fig. 2A and B), and as CCNG2-negative when the cells exhibited a cytoplasmic pattern (no staining in the nucleus; Fig. 2C) or a negative pattern (no staining in the nucleus or cytoplasm; Fig. 2D) in the pancreatic cancer lesions. Among the 36 samples examined, 17 samples (47.2%) were positive for CCNG2, whereas 19 samples (52.8%) were negative.
Figure 1.
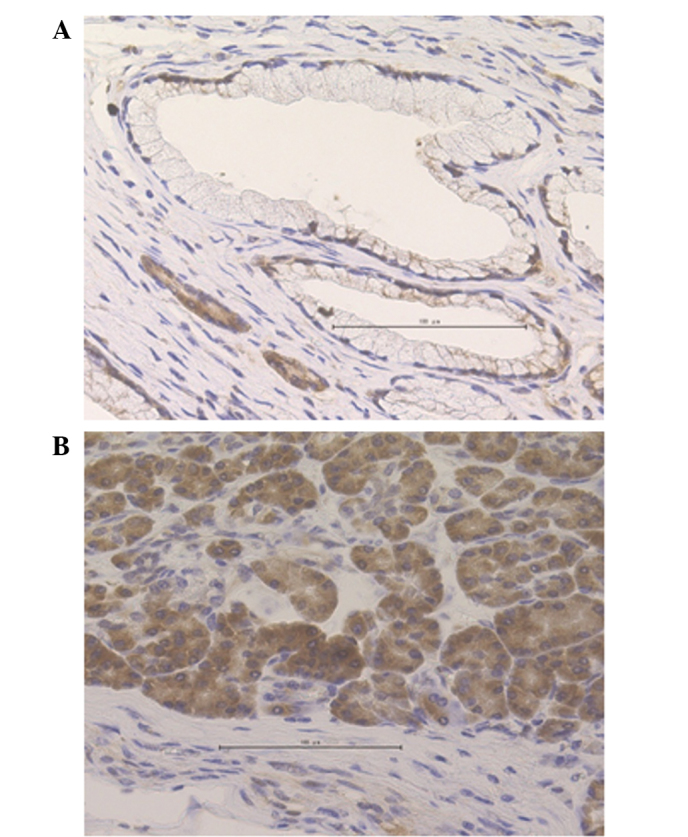
Expression levels of cyclin G2 in the normal section of pancreatic tissues demonstrated by immunohistochemical staining. (A) Normal pancreatic ductal cells and (B) acinar cells that were used as positive controls.
Figure 2.
Cyclin G2 (CCNG2) expression in primary pancreatic cancer samples, observed by immunohistochemical staining. CCNG2-positive cases demonstrated a (A) diffused or (B) spotted nuclear pattern; by contrast, CCNG2-negative cases exhibited a (C) cytoplasmic pattern (no staining in the nucleus) or (D) negative pattern (no staining in the nucleus or cytoplasm).
CCNG2 expression and clinicopathological characteristics
The clinical and histopathological factors between the CCNG2-positive and CCNG2-negative patients were compared to examine the correlation between CCNG2 expression and cancer progression (Table II). The histopathological analysis revealed that venous invasion and the tumor invasion depth (pT) factor (18) were significantly higher in the CCNG2-negative group compared with the CCNG2-positive group. The pathological stage also tended to be higher in the CCNG2-negative group. Therefore, CCNG2 expression correlated inversely with cancer progression in pancreatic cancer.
Table II.
Comparison of clinical and histopathological factors between the CCNG2-positive and -negative groups.
| Characteristics | CCNG2-positive (n=17) | CCNG2-negative (n=19) | P-value |
|---|---|---|---|
| Mean age, years | 68.6±8.6 | 68.4±10.4 | NS |
| Gender (male/female), n | 9/8 | 12/7 | NS |
| Tumor location (Ph/Pb/Pt), n | 8/5/4 | 13/5/1 | NS |
| Maximal diameter, mm | 26.6±19.5 | 25.4±8.6 | NS |
| Histology (well/mod/poor), n | 2/12/3 | 0/18/1 | NS |
| Lymphatic invasion (+/-), n | 10/7 | 16/3 | NS |
| Venous invasion (+/-), n | 4/13 | 11/8 | 0.0489 |
| Intrapancreatic perineural invasion (+/-), n | 12/5 | 17/2 | NS |
| pT (T1/T2/T3/T4), n | 4/3/10/0 | 0/1/18/0 | 0.0242 |
| pN (+/-), n | 6/11 | 10/9 | NS |
| pStage (IA/IB/IIA/IIB/III/IV), n | 4/3/4/6/0/0 | 0/1/8/10/0/0 | 0.0728 |
| Adjuvant therapy (+/-), n | 11/6 | 10/9 | NS |
| Recurrence (+/-), n | 7/10 | 12/7 | NS |
CCNG2, cyclin G2; NS, not significant; Ph/Pb/Pt, pancreatic head/body/tail; (+/-), yes/no; well/mod/poor, well/moderately/poorly-differentiated; pT, tumor invasion depth; pN, lymph node metastasis.
Association between CCNG2 expression and prognosis
Predictive markers for overall survival were assessed based on the clinicopathological details of the patients. Upon univariate analysis, pT, lymph node metastasis (pN) (18) and CCNG2 expression were found to be significantly associated with overall survival, as opposed to other prognostic markers (Table III). Furthermore, multivariate analysis identified pN and CCNG2 expression as significant and independent prognostic factors.
Table III.
Predictive markers for overall survival in the clinicopathological information.
| Parameter | Univariate analysis HR (95% CI) | P-value | Multivariate analysis HR (95% CI) | P-value |
|---|---|---|---|---|
| Mean age (≥69/<69 years) | 2.04 (0.79–5.88) | 0.143 | – | – |
| Gender (male/female) | 1.18 (0.47–3.19) | 0.722 | – | – |
| Maximal diameter (≥26/<26 mm) | 0.84 (0.27–2.19) | 0.731 | – | – |
| Histology (well/mod/poor) | 1.95 (0.40–35.23) | 0.472 | – | – |
| pT (T1, T2/T3, T4) | 0.15 (0.023–0.55) | 0.003 | 0.22 (0.026–1.30) | 0.095 |
| pN (+/-) | 4.15 (1.62–11.08) | 0.003 | 3.18 (1.08–10.45) | 0.035 |
| Lymphatic invasion (+/-) | 1.86 (0.67–6.54) | 0.245 | ||
| Venous invasion (+/-) | 2.35 (0.94–6.11) | 0.066 | 2.43 (0.69–8.18) | 0.159 |
| Intrapancreatic perineural invasion (+/-) | 4.41 (0.91–79.44) | 0.070 | 3.33 (0.54–64.60) | 0.217 |
| CCNG2 expression (+/-) | 0.32 (0.11–0.84) | 0.020 | 0.32 (0.096–0.98) | 0.046 |
HR, hazard ratio; CI, confidence interval; well/mod/poor, well/moderately/poorly-differentiated; pT, tumor invasion depth; pN, lymph node metastasis; (+/-), yes/no or positive/negative; CCNG2, cyclin G2.
The Kaplan-Meier curve for overall survival is shown in Fig. 3 and reveals that the negative expression of CCNG2 was a consistent indicator of poor prognosis in pancreatic cancer patients. The median survival time of the CCNG2-negative group was 19.6 months, whereas that of the CCNG2-positive group was 47.9 months.
Figure 3.
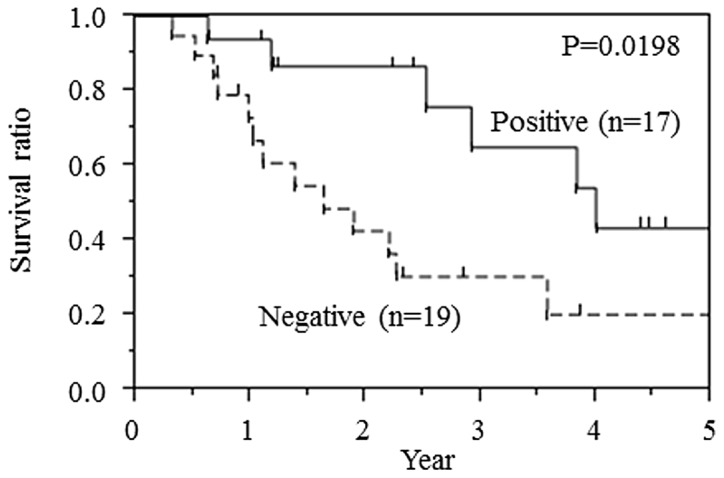
Overall survival rate of 36 patients who underwent complete surgical resection for pancreatic cancer with clear histological margins, according to cyclin G2 expression.
Discussion
CCNG2 gene was initially identified in 1996 and encodes for a protein that belongs to a family of cyclins homologous to CCNG1 (7). Previous studies have reported that CCNG2 participates in carcinogenesis and is a known tumor suppressor gene (15–17,20–26). CCNG2 gene expression is downregulated in thyroid (20), oral (21), ovarian (22), breast (23,24), gastric (16), esophageal (17), prostate (25), kidney (26) and colorectal (15) cancer cells.
Several aspects of CCNG2 behavior are associated with antitumor effects. Antitumor agents induce CCNG2 expression, which results in the inhibition of cancer cell proliferation (8–10). In breast cancer, CCNG2 knockdown induces multidrug resistance (8). In colorectal cancer, CCNG2 expression correlates with the tumor stage, lymph node metastasis, clinical stage, histological grade and overall survival (15). In gastric cancer, CCNG2 expression correlates with the extent of differentiation: CCNG2 expression is high in well-differentiated adenocarcinomas and low in poorly-differentiated adenocarcinomas (12). In our previous study, CCNG2 induced apoptosis and was partially associated with cancer stemness in a pancreatic cancer cell line (13). In summary, CCNG2 is heavily involved in cancer progression, including proliferation, invasion, chemoresistance and differentiation in various cancer types.
In the present study, several histopathological factors associated with clinical outcome were evaluated. CCNG2 was identified as an independent novel prognostic factor in pancreatic cancer patients. In the CCNG2-negative group, the rate of venous invasion and pT factor were significantly higher, while the pathological stage was also higher compared with that in the positive group. Altogether, these findings suggest that low expression of CCNG2 reflects, at least partially, cancer progression and CCNG2 is an independent prognostic factor.
In conclusion, the present study demonstrated that CCNG2 expression correlates inversely with cancer progression and may be used as a novel, independent prognostic factor in pancreatic cancer.
Acknowledgements
The authors would like to thank the members of our laboratories for their contribution. This study was supported in part by: A Grant-in-Aid for Scientific Research and a grant from the Platform for Drug Discovery, Informatics, and Structural Life Science, from the Ministry of Education, Culture, Sports, Science and Technology (grant nos. 23390199, 25112708, 25134711, 30253420, 26670604; P-Direct); a Grant-in-Aid from the Third Comprehensive 10-year Strategy for Cancer Control, Ministry of Health, Labor, and Welfare (grant no. H23-003); a grant from the Kobayashi Cancer Research Foundation; a grant from the Princess Takamatsu Cancer Research Fund, Japan; and a grant from the National Institute of Biomedical Innovation, Japan.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi H, Nagano H, Tanemura M, Takeda Y, Marubashi S, Kobayashi S, Kawamoto K, Wada H, Hama N, Akita H, et al. Preoperative chemoradiotherapy, surgery and adjuvant therapy for resectable pancreatic cancer. Hepatogastroenterology. 2013;60:904–911. doi: 10.5754/hge12974. [DOI] [PubMed] [Google Scholar]
- 5.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 6.Ohigashi H, Ishikawa O, Eguchi H, Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H, Tomita Y, et al. Feasibility and efficacy of combination therapy with preoperative full-dose gemcitabine, concurrent three-dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg. 2009;250:88–95. doi: 10.1097/SLA.0b013e3181ad65cc. [DOI] [PubMed] [Google Scholar]
- 7.Bates S, Rowan S, Vousden KH. Characterisation of human cyclin G1 and G2: DNA damage inducible genes. Oncogene. 1996;13:1103–1109. [PubMed] [Google Scholar]
- 8.Kasukabe T, Okabe-Kado J, Honma Y, Cotylenin A. a new differentiation inducer and rapamycin cooperatively inhibit growth of cancer cells through induction of cyclin G2. Cancer Sci. 2008;99:1693–1698. doi: 10.1111/j.1349-7006.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padua MB, Hansen PJ. Changes in expression of cell-cycle-related genes in PC-3 prostate cancer cells caused by ovine uterine serpin. J Cell Biochem. 2009;107:1182–1188. doi: 10.1002/jcb.22222. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Liu Y, He H, Chen X, Chen J, Lu YC. Candidate genes influencing sensitivity and resistance of human glioblastoma to Semustine. Brain Res Bull. 2011;86:189–194. doi: 10.1016/j.brainresbull.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Naito Y, Yabuta N, Sato J, Ohno S, Sakata M, Kasama T, Ikawa M, Nojima H. Recruitment of cyclin G2 to promyelocytic leukemia nuclear bodies promotes dephosphorylation of γH2AX following treatment with ionizing radiation. Cell Cycle. 2013;12:1773–1784. doi: 10.4161/cc.24878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi MG, Noh JH, An JY, Hong SK, Park SB, Baik YH, Kim KM, Sohn TS, Kim S. Expression levels of cyclin G2, but not cyclin E, correlate with gastric cancer progression. J Surg Res. 2009;157:168–174. doi: 10.1016/j.jss.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa S, Eguchi H, Nagano H, Konno M, Tomimaru Y, Wada H, Hama N, Kawamoto K, Kobayashi S, Nishida N, et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br J Cancer. 2014;111:1572–1580. doi: 10.1038/bjc.2014.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li WJ, Liu GL, Yu F, Xiang XX, Lu YF, Xiao HZ, Shi YP. CCNG2 suppressor biological effects on thyroid cancer cell through promotion of CDK2 degradation. Asian Pac J Cancer Prev. 2013;14:6165–6171. doi: 10.7314/APJCP.2013.14.10.6165. [DOI] [PubMed] [Google Scholar]
- 15.Sun GG, Zhang J, Hu WN. CCNG2 expression is downregulated in colorectal carcinoma and its clinical significance. Tumour Biol. 2014;35:3339–3346. doi: 10.1007/s13277-013-1440-5. [DOI] [PubMed] [Google Scholar]
- 16.Sun GG, Hu WN, Cui DW, Zhang J. Decreased expression of CCNG2 is significantly linked to the malignant transformation of gastric carcinoma. Tumour Biol. 2014;35:2631–2639. doi: 10.1007/s13277-013-1346-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen JQ, Liu CJ, Wen HX, Shi CL, Zhang HS, Li M, Sun GG. Changes in the expression of cyclin G2 in esophageal cancer cell and its significance. Tumour Biol. 2014 doi: 10.1007/s13277-013-1442-3. [DOI] [PubMed] [Google Scholar]
- 18.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumors. 7th. Wiley-Blackwell; Oxford: 2009. [Google Scholar]
- 19.Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu J, Eguchi H, Miyamoto A, Dono K, Umeshita K, et al. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin Cancer Res. 1999;5:4005–4012. [PubMed] [Google Scholar]
- 20.Ito Y, Yoshida H, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Kuma K, et al. Decreased expression of cyclin G2 is significantly linked to the malignant transformation of papillary carcinoma of the thyroid. Anticancer Res. 2003;23:2335–2338. [PubMed] [Google Scholar]
- 21.Kim Y, Shintani S, Kohno Y, Zhang R, Wong DT. Cyclin G2 dysregulation in human oral cancer. Cancer Res. 2004;64:8980–8986. doi: 10.1158/0008-5472.CAN-04-1926. [DOI] [PubMed] [Google Scholar]
- 22.Fu G, Peng C. Nodal enhances the activity of FoxO3a and its synergistic interaction with Smads to regulate cyclin G2 transcription in ovarian cancer cells. Oncogene. 2011;30:3953–3966. doi: 10.1038/onc.2011.127. [DOI] [PubMed] [Google Scholar]
- 23.Montagner M, Enzo E, Forcato M, Zanconato F, Parenti A, Rampazzo E, Basso G, Leo G, Rosato A, Bicciato S, et al. SHARP1 suppresses breast cancer metastasis by promoting degradation of hypoxia-inducible factors. Nature. 2012;487:380–384. doi: 10.1038/nature11207. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed S, Al-Saigh S, Matthews J. FOXA1 is essential for aryl hydrocarbon receptor-dependent regulation of cyclin G2. Mol Cancer Res. 2012;10:636–648. doi: 10.1158/1541-7786.MCR-11-0502. [DOI] [PubMed] [Google Scholar]
- 25.Cui DW, Cheng YJ, Jing SW, Sun GG. Effect of cyclin G2 on proliferative ability of prostate cancer PC-3 cell. Tumour Biol. 2014;35:3017–3024. doi: 10.1007/s13277-013-1389-4. [DOI] [PubMed] [Google Scholar]
- 26.Cui DW, Sun GG, Cheng YJ. Change in expression of cyclin G2 in kidney cancer cell and its significance. Tumour Biol. 2014;35:3177–3183. doi: 10.1007/s13277-013-1415-6. [DOI] [PubMed] [Google Scholar]