Abstract
BACKGROUND:
Indwelling arterial catheters (IACs) are used extensively in the ICU for hemodynamic monitoring and for blood gas analysis. IAC use also poses potentially serious risks, including bloodstream infections and vascular complications. The purpose of this study was to assess whether IAC use was associated with mortality in patients who are mechanically ventilated and do not require vasopressor support.
METHODS:
This study used the Multiparameter Intelligent Monitoring in Intensive Care II database, consisting of > 24,000 patients admitted to the Beth Israel Deaconess Medical Center ICU between 2001 and 2008. Patients requiring mechanical ventilation who did not require vasopressors or have a diagnosis of sepsis were identified, and the primary outcome was 28-day mortality. A model based on patient demographics, comorbidities, vital signs, and laboratory results was developed to estimate the propensity for IAC placement. Patients were then propensity matched, and McNemar test was used to evaluate the association of IAC with 28-day mortality.
RESULTS:
We identified 1,776 patients who were mechanically ventilated who met inclusion criteria. There were no differences in the covariates included in the final propensity model between the IAC and non-IAC propensity-matched groups. For the matched cohort, there was no difference in 28-day mortality between the IAC group and the non-IAC group (14.7% vs 15.2%; OR, 0.96; 95% CI, 0.62-1.47).
CONCLUSIONS:
In hemodynamically stable patients who are mechanically ventilated, the presence of an IAC is not associated with a difference in 28-day mortality. Validation in other datasets, as well as further analyses in other subgroups, is warranted.
Indwelling arterial catheters (IACs) are used in the ICU setting for continuous hemodynamic monitoring and for arterial blood sampling for blood gas analysis. IAC use in the ICU setting is widespread, occurring in approximately 30% of all patients in the ICU, with relatively stable IAC use over time.1‐3
Despite widespread IAC use, there are rare but potentially serious complications that may arise. IAC-associated bloodstream infections have been reported at a rate that, although not to the level of central venous catheters, is significantly higher than peripheral venous access. A systematic review of the risk of bloodstream infections associated with intravascular catheters reports a pooled point estimate of 1.6 per 1,000 device days (95% CI, 1.2-2.3) for IAC compared with 0.5 (95% CI, 0.2-0.7) for peripheral venous access and 2.7 (95% CI, 2.6-2.9) for central venous catheters.4 Additionally, vascular complications associated with IAC use are more common than previously believed, including thrombosis, ischemia, hematoma, bleeding, and pseudoaneurysm.5 The presence of an IAC may promote increased frequency of blood draws and laboratory testing, including arterial blood gas sampling.6,7
In the context of increased IAC-associated use and complications, there are scant outcomes data to support their widespread use. The purpose of this study was to examine the association between IAC use and outcomes in a large cohort of hemodynamically stable intensive care patients with respiratory failure undergoing mechanical ventilation.
Materials and Methods
Study Population
We conducted a longitudinal, single-center, retrospective cohort study of patients from the Multiparameter Intelligent Monitoring of Intensive Care (MIMIC-II) database, which includes patients admitted between 2001 and 2008. The database contains data from 24,581 patients in ICUs and includes physiologic information from bedside monitors and hospital information systems in the adult ICUs at Beth Israel Deaconess Medical Center, a tertiary care university academic medical center located in Boston, Massachusetts.8 The data in MIMIC-II has been previously deidentified, and the institutional review boards of the Massachusetts Institute of Technology (No. 0403000206) and Beth Israel Deaconess Medical Center (2001-P-001699/14) both approved the use of the database for research.
The MIMIC-II database was searched to identify adult patients requiring mechanical ventilation within the first 24 h of medical or surgical ICU admission and lasting for at least 24 h. The presence of an IAC was defined as placement of an invasive arterial catheter at any point in time after initiation of mechanical ventilation. Patients were excluded if they had a diagnosis of sepsis based on the Angus criteria9 or required vasopressors while in the ICU or if IAC placement was performed prior to endotracheal intubation and initiation of mechanical ventilation (including pre-ICU admission IAC placement). As the majority of patients in the cardiac surgery recovery unit had an IAC placed prior to ICU arrival, all patients from the cardiac surgery ICU were also excluded from this analysis. Additionally, to ensure the independence of data, only the first ICU admission was included in patients who had multiple ICU admissions.
Coincident diseases were obtained based on International Classification of Diseases, Ninth Revision, Clinical Modification. The Sequential Organ Failure Assessment (SOFA) score was obtained at the time of ICU admission, and laboratory values immediately preceding onset of mechanical ventilation were used.
Outcome Measures
The primary outcome was 28-day mortality. Secondary outcomes included ICU and hospital length of stay (LOS), duration of mechanical ventilation, and mean number of arterial and venous blood gas measurements performed per day while admitted to the ICU.
Statistical Analysis
A propensity score model was created to match baseline patient characteristics. Twenty-nine pre-IAC placement features, including patient demographics, comorbidities, vital signs, and preintervention laboratory results, were selected from 53 available candidate variables (those without significant missing data) to estimate the propensity for IAC insertion using a genetic algorithm (e-Appendix 1 (486.1KB, pdf) ).10 Patients with or without IAC placement were then matched based on the estimated propensity scores using one-to-one matching without replacement with a caliper of 0.01. To ensure the robustness of the propensity score model and to avoid over-fitting, the goodness-of-fit of the prediction model was evaluated based on the average area under the receiver operating characteristic curve using 10-fold cross-validation, and the predictive model was also evaluated with the Hosmer-Lemeshow test.
The success of the propensity score model was evaluated by assessment of the differences in baseline covariates between IAC and non-IAC groups. As continuous variables were not normally distributed, median values and interquartile range (IQR) were used to summarize distributions. The Fisher exact test and Wilcoxon rank-sum test were applied to statistically assess the differences in categorical and continuous variables between the unmatched IAC and non-IAC groups. Measures of association for baseline covariates in the propensity-matched cohorts were performed using either McNemar test for categorical variables or Wilcoxon signed rank test for continuous variables. The distributions of the propensity score before and after matching were also compared to further assess the degree of balance.
In univariate analyses, a McNemar test was performed for binary outcomes and paired t tests for continuous outcomes. As mortality is a competing risk for ICU LOS, total LOS, and duration of mechanical ventilation, we used the cumulative incidence function to estimate the probability of the secondary outcome over 28 days while allowing for the possibility of alternative outcomes (eg, death) to occur.11
Sensitivity Analyses
Sensitivity analyses were performed to evaluate the effects of varying both the inclusion criteria of time to mechanical ventilation (to include all patients undergoing endotracheal intubation at any point during their ICU course) and the caliper level for propensity matching on the association between IAC placement and 28-day mortality. Ten different caliper levels between 0.01 and 0.1 at 0.01 increments were used to match the positive and negative controls. We also performed a sensitivity analysis using propensity score weights to create an alternative propensity score model for IAC placement. This method optimizes postweighting balance of covariates between groups, and a weighted regression model including any imbalanced covariates between the matched groups was estimated for 28-day mortality (e-Appendix 1 (486.1KB, pdf) ).
Results
Propensity Score Matching
Of the 24,581 MIMIC-II admissions reviewed, 24,443 patients remained after eliminating multiple admissions. A total of 1,776 patients met inclusion criteria (Fig 1), of whom 44.6% had an IAC. Figure 2 shows the distribution of the propensity score of the IAC and the non-IAC groups before and after matching. The propensity score model for IAC placement yielded 0.79 for the area under the receiver operating characteristic curve (> 10-fold cross-validation) and a P value of .83 for the Hosmer-Lemeshow test. After 1:1 matching, the propensity-matched sample consisted of 696 patients (348 patients with respiratory failure who underwent IAC placement matched to 348 patients with respiratory failure who do not have an IAC placed). In the matched cohort, the median ages for the IAC and non-IAC groups were 54 years (IQR, 38-73 years) and 53 years (IQR, 35-72 years), respectively. There were no differences between the IAC and non-IAC propensity-matched groups for covariates included in the final propensity score model, including chronic comorbidities and acute respiratory diagnoses such as ARDS and pneumonia (Table 1; e-Figs 1, 2 (486.1KB, pdf) ; e-Tables 1-4 (486.1KB, pdf) ).
Figure 1 –
Flowchart of patient inclusion. CSRU = cardiac surgery recovery unit; IAC = indwelling arterial catheter; MIMIC-II = Multiparameter Intelligent Monitoring in Intensive Care-II.
Figure 2 –
A-D, Propensity score distribution plot comparing non-IAC (A, C) and IAC (B, D) groups before (A, B) and after (C, D) matching. See Figure 1 legend for expansion of abbreviation.
TABLE 1 ] .
Baseline Covariates Between IAC and Non-IAC Groups in Unmatched Cohorts and Propensity-Matched Cohorts
| Variables | Entire Cohort (N = 1,776) | Matched Cohort (N = 696) | ||||
| Non-IAC (n = 984) | IAC (n = 792) | P Value | Non-IAC (n = 348) | IAC (n = 348) | P Value | |
| Age, y | 51 (35-72) | 56 (40-73) | .01 | 53 (35-72) | 54 (38-73) | .8 |
| Female | 344 (43.5) | 406 (41.3) | .36 | 205 (58.9) | 192 (55.2) | .6 |
| SOFA score | 5 (4-6) | 6 (5-8) | < .0001 | 5 (4-7) | 6 (4-7) | .5 |
| Service unit | < .0001 | .3 | ||||
| MICU | 504 (63.6) | 290 (29.5) | 184 (52.9) | 192 (55.2) | ||
| SICU | 288 (26.4) | 694 (70.5) | 164 (47.1) | 156 (44.8) | ||
| Coincident diseases | ||||||
| COPD | 81 (10.2) | 76 (7.7) | .07 | 32 (9.2) | 39 (11.2) | .8 |
| Respiratory disease (non-COPD)a | 278 (35.1) | 287 (29.2) | .01 | 121 (34.7) | 125 (35.9) | .5 |
| Pneumonia | 147 (18.6) | 152 (15.5) | .01 | 67 (20.0) | 68 (20.3) | .7 |
| Congestive heart failure | 97 (12.5) | 116 (11.8) | .70 | 44 (12.6) | 36 (10.3) | .6 |
| Atrial fibrillation | 82 (10.4) | 125 (12.7) | .10 | 36 (10.3) | 32 (9.2) | 1 |
| Chronic kidney disease | 28 (3.5) | 32 (3.3) | .80 | 13 (3.8) | 10 (2.9) | 1 |
| Chronic liver disease | 28 (4.8) | 61 (6.2) | .20 | 14 (4.0) | 18 (5.2) | .7 |
| Coronary artery disease | 51 (6.4) | 72 (7.32) | .50 | 23 (6.6) | 21 (6.0) | .2 |
| Stroke | 70 (8.8) | 152 (15.5) | .0001 | 32 (9.2) | 33 (9.5) | .9 |
| Malignancy | 92 (11.6) | 164 (16.7) | .003 | 44 (12.6) | 51 (14.7) | .4 |
| Laboratory tests | ||||||
| WBC | 10.6 (7.8-14.3) | 11.8 (8.5-15.9) | < .0001 | 10.7 (8.0-14.8) | 11.5 (8.4-14.7) | .8 |
| Hemoglobin | 13 (11.3-14.4) | 12.6 (11-14.1) | .003 | 12.8 (11.2 -14.2) | 12.7 (11.0-14.1) | .8 |
| Platelet | 246 (190-304) | 237 (177-294) | .01 | 238 (184-303) | 238 (186-289) | .8 |
| Sodium | 140 (138-143) | 140 (137-142) | .01 | 140 (138-143) | 140 (137-142) | .6 |
| Potassium | 4 (3.6-4.5) | 4 (3.7-4.4) | .77 | 4 (3.6-4.5) | 4 (3.7-4.4) | .9 |
| Bicarbonate | 24 (22-27) | 24 (21-27) | .05 | 24 (22-27) | 24 (21-27) | .3 |
| Chloride | 104 (100-107) | 104 (101-108) | .0003 | 104 (100-107) | 104 (100-107) | .3 |
| BUN | 15 (11-21) | 16 (12-22) | .02 | 15 (11-22) | 16 (12-22) | .7 |
| Creatinine | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) | .60 | 0.9 (0.7-1.2) | 0.9 (0.7-1.1) | .6 |
| Po2 | 206 (96-375) | 200 (108-337) | .50 | 180 (104-340) | 187 (106-300) | .8 |
| Pco2 | 42 (37-50) | 41 (36-48) | .02 | 41.5 (37-47) | 40 (35-47) | .6 |
| DNR at admission | 65 (8.2) | 39 (4.0) | < .0001 | 20 (5.8) | 12 (3.5) | .6 |
| Change in code status during ICU admissionb | 41 (5.2) | 95 (9.7) | < .0001 | 35 (10.4) | 34 (10.1) | .9 |
Data presented as No. (%) or median (interquartile range). DNR = do not resuscitate; IAC = indwelling arterial catheter; MICU = medical ICU; SICU = surgical ICU; SOFA = Sequential Organ Failure Assessment.
International Classification of Diseases, Ninth Revision, Clinical Modification code 518*, which includes ARDS.
Defined as code status change to Do Not Resuscitate or Comfort Measures Only.
Primary and Secondary Outcomes
After propensity score matching, there was no difference in 28-day mortality in the IAC (14.7%) vs non-IAC (15.2%) groups (OR, 0.96; 95% CI, 0.62-1.47) (Table 2). Patients with an IAC had a significantly lower likelihood of discharge from the ICU (P < .0001; sub-hazard ratio [HR], 0.72; 95% CI, 0.61-0.86) or from the hospital (P < .0001; sub-HR, 0.71; 95% CI, 0.60-0.84) at 28 days. Likewise, patients with an IAC had a lower likelihood of successful ventilator removal (P < .0001; sub-HR, 0.74; 95% CI, 0.63-0.87) at 28 days. When survivors were separately analyzed, ICU LOS, hospital LOS, and duration of mechanical ventilation were significantly shorter among patients without an IAC (Table 2). Patients with an IAC had a mean difference of 1.44 more blood gas measurements performed per day (P < .0001).
TABLE 2 ] .
Primary and Secondary Outcomes for Propensity-Matched IAC and Non-IAC Groups
| Outcomes | Non-IAC | IAC | P Value | Effect Size |
| Primary outcome | ||||
| 28-d mortality | 15.2% | 14.7% | .83 | 0.96 (0.62-1.47)a |
| Secondary outcomes, mean (SD) | ||||
| ICU LOS (survivors) | 2.2 (1.4) | 3.7 (3.1) | < .0001 | 1.65 (1.24-2.07)b |
| Hospital LOS (survivors) | 5.7 (4.8) | 9.4 (7.5) | < .0001 | 3.47 (2.34-4.59)b |
| Mechanical ventilation time (survivors) | 1.0 (1.0) | 2.1 (2.6) | < .0001 | 1.10 (0.76-1.42)b |
| Blood gas measurements (per 24 h) | 1.0 (0.8) | 2.4 (1.4) | < .0001 | 1.44 (1.27-1.62)b |
Data presented as % or mean (SD). LOS = length of stay. See Table 1 legend for expansion of other abbreviation.
OR (95% CI).
Standardized mean difference (95% CI).
Sensitivity Analyses
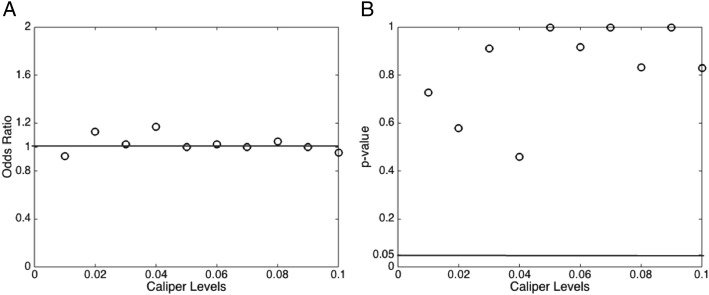
The study cohort only included patients who were intubated within 24 h of admission to the ICU. We performed a sensitivity analysis that included all patients who were intubated regardless of timing. No significant difference in 28-day mortally between the IAC and non-IAC group (P = .4) was observed in this expanded cohort. Figure 3 summarizes the results of the sensitivity analyses using various matching caliper levels. As shown in Figure 3A, the ORs for IAC placement and 28-day mortality are around 1.0 for all caliper levels. As shown in Figure 3B, measures of association for all caliper levels did not reach statistical significance (P > .05). Using the propensity score weight methodology, there remained no difference in 28-day mortality between the IAC and non-IAC groups (e-Appendix 1 (486.1KB, pdf) ).
Figure 3 –
Sensitivity analyses of various matching caliper levels. A, ORs. B, P values.
Discussion
In this propensity-matched cohort analysis of hemodynamically stable patients who were mechanically ventilated, we report no association between the placement of an invasive arterial catheter and 28-day mortality. Placement of an IAC was, however, associated with a longer duration of mechanical ventilation, ICU and hospital LOS, and an increased frequency of blood gas sampling after matching patients for propensity to receive an IAC.
There are several potential explanations for the lack of association between IAC use and mortality in our analysis. First, the blood gas data and hemodynamic measurements obtained from IAC do not provide valuable clinical data that lead to changes in management that translate into a measurable impact on mortality. Alternatively, the results of this analysis may be attributed to unmeasured confounding, which we attempted to account for by using a propensity-matched cohort. Our findings from the MIMIC-II database are consistent with a recent study using the Project IMPACT database, which reported no association between IAC and mortality in patients in the ICU.12 Our findings support the need for replication in additional large critical care databases as well as future randomized controlled trials (RCTs) to investigate causation between IAC and patient outcomes.
The care of critically ill patients is an excellent case study in the adoption of technological advancement within health care. An example of this is the use of pulmonary arterial catheters in critically ill patients, which was a widely accepted and used monitoring device before 13 subsequent randomized clinical trials and repeated meta-analyses demonstrating no improvement in patient outcomes13,14 led to subsequent declines in pulmonary arterial catheter use over time.15,16 Despite lessons learned, IAC use remains common, and in recent years the development and use of other invasive and noninvasive modalities of hemodynamic monitoring has increased to include arterial waveform analysis, bedside echocardiography, esophageal Doppler, and noninvasive bioimpedance/bioreactance, all with limited to no demonstrated benefit in patient outcomes. RCTs to investigate causal relationships between these monitoring devices and outcomes within specific patient subsets and clinical contexts are warranted, although there are often cost and logistical challenges to performing RCTs in the ICU. Research using highly granular databases such as MIMIC-II should be explored to identify subpopulations of critically ill patients who may benefit from specific technology application, thus allowing for more focused RCTs and more parsimonious application of technology.
Additionally, the MIMIC-II database contains comprehensive electronic health record data throughout the hospital course. Our analysis leverages the availability of time-stamped vital signs, laboratory results, and interventions to build a propensity score model by including predictors and confounders available at the time the clinical decision was made. Such granularity is important in creating propensity score models at the time when the decisions are made, especially in a highly dynamic setting such as the ICU. The granularity of these data are also particularly useful for decision analysis, evaluation of information gain, personalized dosage calculation,17 or comparative effectiveness studies,18 which have been traditionally performed using low-resolution data.
There are several limitations, however, that should be noted. First, as this is a single-center study from an academic tertiary care center, our findings may not be generalizable to other institutions. Residual confounding may also mar our findings, although we attempted to account for this through propensity matching. Potential unmeasured confounders not accounted for in this analysis include relevant past medical history, such as prior episodes of respiratory failure or prolonged mechanical ventilation, as well as treating physician(s). This raises the possibility that there may be negative confounding that contributed to our findings of no association between IAC placement and mortality. Additionally, the potential for immortal time bias and indication bias is present, as in all observational studies. We attempted to minimize interaction or effect modification by limiting our primary analysis to patients admitted to the ICU with acute respiratory failure without hemodynamic compromise requiring vasopressor support or concomitant sepsis, which are alternative reasons IAC placement may be considered. By limiting our study sample to a single indication for IAC placement, we are also attempting to optimize our propensity score model for assessment of IAC placement and 28-day mortality. There will be different relationships between covariates, IAC placement, and 28-day mortality based on indication for IAC placement, which will have effects on bias, variance, and mean squared error of the estimated exposure effect.19 Of note, we plan on performing subsequent analyses in MIMIC-II and larger electronic health record-derived datasets for other ICU subgroups with different indications for IAC placement. We are unable to report potential adverse events associated with IAC placement and use, including catheter-associated bloodstream infections or vascular complications, as these were not consistently captured in MIMIC-II. Finally, although our findings do not support an association between IAC use and mortality, only RCTs can establish a causal relationship.
Conclusions
In this single-center, retrospective study of hemodynamically stable patients requiring mechanical ventilation, the placement of invasive arterial catheters was not associated with a change in mortality as compared with propensity-matched patients without invasive arterial catheters. Invasive arterial catheters were associated with an increased ICU LOS, total LOS, duration of mechanical ventilation, and increased blood gas measurements.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: L. A. C. was the principal investigator, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. D. J. H., M. F., and L. A. C. contributed to conception and design and D. J. H., M. F., R. K., H. Z., K. P. C., and L. A. C. contributed to analysis, data collection and interpretation, and drafting the manuscript.
Conflict of interest: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Material section of the online article.
ABBREVIATIONS
- HR
hazard ratio
- IAC
indwelling arterial catheter
- IQR
interquartile range
- LOS
length of stay
- MIMIC-II
Multiparameter Intelligent Monitoring in Intensive Care-II
- RCT
randomized controlled trial
Footnotes
Drs Hsu and Feng contributed equally to this work.
FUNDING/SUPPORT: This study was supported by the National Institute of Biomedical Imaging and Bioengineering [Grant R01 EB001659]. Dr Feng is supported by an A*STAR Graduate Scholarship.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA; Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34(4):1016-1024. [DOI] [PubMed] [Google Scholar]
- 2.Gershengorn HB, Garland A, Kramer A, Scales DC, Rubenfeld G, Wunsch H. Variation of arterial and central venous catheter use in United States intensive care units. Anesthesiology. 2014;120(3):650-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traoré O, Liotier J, Souweine B. Prospective study of arterial and central venous catheter colonization and of arterial- and central venous catheter-related bacteremia in intensive care units. Crit Care Med. 2005;33(6):1276-1280. [DOI] [PubMed] [Google Scholar]
- 4.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159-1171. [DOI] [PubMed] [Google Scholar]
- 5.Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6(3):199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low LL, Harrington GR, Stoltzfus DP. The effect of arterial lines on blood-drawing practices and costs in intensive care units. Chest. 1995;108(1):216-219. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman JE, Seneff MG, Sun X, Wagner DP, Knaus WA. Evaluating laboratory usage in the intensive care unit: patient and institutional characteristics that influence frequency of blood sampling. Crit Care Med. 1997;25(5):737-748. [DOI] [PubMed] [Google Scholar]
- 8.Scott DJ, Lee J, Silva I, et al. Accessing the public MIMIC-II intensive care relational database for clinical research. BMC Med Inform Decis Mak. 2013;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310. [DOI] [PubMed] [Google Scholar]
- 10.Diamond A, Sekhon JS. Genetic matching for estimating causal effects: a general multivariate matching method for achieving balance in observational studies. Rev Econ Stat. 2013;95(3):932-945. [Google Scholar]
- 11.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ: John Wiley & Sons, Ltd; 2002. [Google Scholar]
- 12.Gershengorn HB, Wunsch H, Scales DC, Zarychanski R, Rubenfeld G, Garland A. Association between arterial catheter use and hospital mortality in intensive care units. JAMA Intern Med. 2014;174(11):1746-1754. [DOI] [PubMed] [Google Scholar]
- 13.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294(13):1664-1670. [DOI] [PubMed] [Google Scholar]
- 14.Rajaram SS, Desai NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013;2:CD003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993-2004. JAMA. 2007;298(4):423-429. [DOI] [PubMed] [Google Scholar]
- 16.Gershengorn HB, Wunsch H. Understanding changes in established practice: pulmonary artery catheter use in critically ill patients. Crit Care Med. 2013;41(12):2667-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghassemi MM, Richter SE, Eche IM, Chen TW, Danziger J, Celi LA. A data-driven approach to optimized medication dosing: a focus on heparin. Intensive Care Med. 2014;40(9):1332-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghassemi M, Celi LA, Stone DJ. State of the art review: the data revolution in critical care. Crit Care. 2015;19:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement