Abstract
Objectives
The objectives of this study were to: (1) develop a novel adhesive for prevention of tooth root caries and secondary caries by possessing a combination of protein-repellent, antibacterial, and remineralization capabilities for the first time; and (2) investigate the effects of 2-methacryloyloxyethyl phosphorylcholine (MPC), dimethylaminohexadecyl methacrylate (DMAHDM), and nanoparticles of amorphous calcium phosphate (NACP) on dentine bond strength, protein-repellent properties, and dental plaque microcosm biofilm response.
Methods
MPC, DMAHDM and NACP were added into Scotchbond Multi-Purpose primer and adhesive. Dentine shear bond strengths were measured. Adhesive coating thickness, surface texture and dentine-adhesive interfacial structure were examined. Protein adsorption onto adhesive resin surface was determined by the micro bicinchoninic acid method. A human saliva microcosm biofilm model was used to investigate biofilm metabolic activity, colony-forming unit (CFU) counts, and lactic acid production.
Results
The resin with 7.5% MPC + 5% DMAHDM + 30% NACP did not adversely affect dentine shear bond strength (p > 0.1). The resin with 7.5% MPC + 5% DMAHDM + 30% NACP produced a coating on root dentine with a thickness of approximately 70 μm and completely sealed all the dentinal tubules. The resin with 7.5% MPC + 5% DMAHDM + 30% NACP had 95% reduction in protein adsorption, compared to SBMP control (p < 0.05). The resin with 7.5% MPC + 5% DMAHDM + 30% NACP was strongly antibacterial, with biofilm CFU being four orders of magnitude lower than that of SBMP control.
Significance
The novel multifunctional adhesive with strong protein-repellent, antibacterial and remineralization properties is promising to coat tooth roots to prevent root caries and secondary caries. The combined use of MPC, DMAHDM and NACP may have wide applicability to bonding agents, cements, sealants and composites to inhibit caries.
Keywords: Dental adhesive, protein repellent, antibacterial, calcium phosphate nanoparticles, dentine bond strength, tooth root caries inhibition
1. Introduction
The prevalence and severity of tooth root caries increases with aging, and this is a growing public health issue due to the rapid increase in the elderly population coupled with substantial increases in tooth retention in seniors [1]. Gingival recession due to aging, periodontal disease or traumatic tooth-brushing habits can increase the susceptibility to root caries [1]. In addition, low salivary flow in seniors and patients with dry mouths further contribute to biofilm and plaque buildup and the occurrence of root caries [2]. Indeed, root caries occurrence in the United States was reported to increase rapidly with aging, from 7% among young people, to 56% in seniors (≥ 75 years of age) [3]. In addition, secondary caries has been suggested in previous studies as a primary reason for dental restoration failures [4,5]. The replacement of the failed restorations accounts for 50–70% of all restorations performed [6]. The annual cost for tooth cavity restorations was approximately $46 billion in 2005 in the United States [7]. Hence, dental caries is a significant burden, and it is highly desirable to develop effective methods to prevent root caries and secondary caries.
Dental plaque formation is a prerequisite for the occurrence of root caries and secondary caries [8,9]. Dental plaque are aggregates of microorganisms, which are formed due to the attachment of bacteria to tooth surface and to each other in the oral environment [9]. Once the tooth root surface or resin restoration surface are exposed in the oral cavity, they are coated with a salivary pellicle that comprises a layer of selectively adsorbed salivary proteins that mediate the binding of microorganisms [8,9]. The adherence of bacteria to the salivary pellicle is an initial step in biofilm formation [8,9]. Therefore, inhibiting protein adsorption and bacterial adherence is a promising approach for suppressing plaque formation and preventing root caries and secondary caries.
The prevention of root caries have been attempted by the daily usage of fluoride solutions or toothpastes [10], professional application of fluoride gel/varnishes [11], or the use of chlorhexidine solutions/varnishes [12]. However, these treatments are temporary and success depends on the compliance of patients [13]. Efficient and simple single-visit methods to prevent root caries are currently not available [14]. Recently, coating of tooth root surface with adhesives was investigated as a preventive treatment against root caries, as it provides a strong physical barrier with the formation of a hybridized layer [14–17]. Besides coating tooth roots, adhesives are also used to bond composite in tooth cavities. However, while composites are the principal material for cavity restorations due to their excellent aesthetics and direct-filling capability [4,5,18–20], resins in vivo tend to accumulate more plaque than other restorative materials [21]. Furthermore, microgaps can be observed at the tooth-restoration interfaces [19,22]. Microleakage can occur and biofilms at the restoration margins can produce acids and cause secondary caries [4,5]. While adhesive compositions and bonding procedures have been improved [23,24], further improvements could be achieved by developing a protein-repellent and antibacterial bonding agent to combat biofilms at the tooth-restoration margins. To date, a dentine adhesive that possesses a combination of protein-repellent and antibacterial capabilities has not been reported.
Quaternary ammonium methacrylates (QAMs) were incorporated into adhesives [25–28]. Antibacterial adhesives reduced biofilm viability and acid production. The antibacterial activity increased when the alkyl chain length (CL) was increased from 5 to 16 [29]. Dentine adhesive containing a new monomer dimethylaminohexadecyl methacrylate (DMAHDM) with CL of 16 had the strongest antibacterial activity [30].
2-methacryloyloxyethyl phosphorylcholine (MPC), a methacrylate with a phospholipid polar group in the side chain, is one of the most common biocompatible and hydrophilic biomedical polymers [31,32]. It has been shown that hydrophilic material surfaces are more resistant to protein adsorption than hydrophobic surfaces [33]. The MPC polymer coating rendered the surfaces extremely hydrophilic and prevented the adsorption of proteins [31,32]. Recently, MPC was incorporated into dentin bonding agents and composites, achieving a strong protein-repellent capability [34,35]. It would be highly desirable to combine MPC with DMAHDM to develop a novel dental adhesive with a combination of protein-repellent and antibacterial capabilities, in order to repel protein and inhibit bacteria attachment, thereby preventing root caries and secondary caries.
Exposure of root dentine after gingival recession is common among seniors because the thin cementum can be lost due to tooth-brushing or biofilm acids [36]. Dentine differs from enamel due to the smaller mineral crystallites with a higher carbonate content [37]. Moreover, dentine mineral is more soluble than enamel mineral [37]. Demineralization on tooth root is approximately twice as rapid as that of enamel [38]. Therefore, it is beneficial for the adhesive that is used to coat tooth roots to also possess remineralization capability. Calcium phosphate (CaP) composites released supersaturating levels of calcium (Ca) and phosphate (P) ions and remineralized tooth lesions in vitro [39]. Recently, nanoparticles of amorphous calcium phosphate (NACP) with a size of 116 nm were synthesized via a spray-drying technique and filled into composites and adhesives [40–44]. These nanocomposites achieved Ca and P ion release similar to those of traditional CaP composites, while possessing much better mechanical properties [40,41].
Previous studies focused on the development of adhesive systems possessing antibacterial and/or remineralization properties [42–44]. To date, there has been no report on the development of a dental adhesive possessing protein-repellent, antibacterial, and Ca and P ion release capabilities. The objective of this study was to develop an adhesive for prevention of root caries and secondary caries by combining MPC with DMAHDM and NACP in the adhesive. Such an adhesive is promising for use in coating tooth roots as well as bonding restorations in tooth cavities. Recent studies showed that dental resins containing NACP remineralized enamel lesions in vitro [41], and inhibited secondary caries in a human in situ study [44]. In the present study, the protein-repellent and antibacterial effects of this adhesive system were evaluated, and its dentin coating and bonding capability was investigated. It was hypothesized that: (1) Incorporating MPC, DMAHDM and NACP into the adhesive would not compromise the dentine bond strength; (2) adhesive with MPC-DMAHDM-NACP would produce hermetic sealing of root dentine with a relatively thick resinous layer as a barrier against biofilm attack; (3) the MPC-DMAHDM-NACP containing adhesive would have much less protein adsorption than commercial control; and (4) the MPC-DMAHDM-NACP containing adhesive would greatly reduce biofilm viability, acid production and colony-forming unit (CFU) counts, compared to commercial control.
2. Materials and methods
2.1. MPC incorporation into bonding agent
Scotchbond multi-purpose (3M, St. Paul, MN), referred to as “SBMP”, was used as the parent bonding system. According to the manufacturer, SBMP etchant contained 35% phosphoric acid. SBMP primer single bottle contained 35–45% 2-hydroxyethylmethacrylate (HEMA), 10–20% copolymer of acrylic and itaconic acids, and 40–50% water. SBMP adhesive contained 60–70% BisGMA and 30–40% HEMA.
MPC (Sigma-Aldrich, St. Louis, MO) was synthesized via a method reported by Ishihara et al [31]. The MPC powder was mixed with SBMP primer at MPC/(SBMP primer + MPC) mass fraction of 7.5%. This mass fraction was selected from previous study showing that 7.5% MPC yielded the strongest protein-repellent property without compromising the dentine bond strength [34]. MPC was added into primer and magnetically-stirred with a bar at a speed of 150 rpm (Bellco Glass, Vineland, NJ) for 24 h to completely dissolve MPC in primer [34]. Similarly, 7.5% of MPC was also mixed into the SBMP adhesive.
2.2. DMAHDM incorporation into bonding agent
DMAHDM was synthesized using a modified Menschutkin reaction where a tertiary amine was reacted with an organo-halide [30]. A benefit of this reaction is that the reaction products are generated at virtually quantitative amounts and require minimal purification. Briefly, 10 mmol of 2-(dimethylamino) ethyl methacrylate (DMAEMA, Sigma-Aldrich) and 10 mmol of 1-bromohexadecane (BHD, TCI America, Portland, OR) were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 70°C for 24 h. The solvent was then removed via evaporation, yielding DMAHDM as a clear, colorless, and viscous liquid [30]. The SBMP primer was first mixed with MPC as described above. Then, DMAHDM was mixed into the SBMP-MPC primer, at DMAHDM/(SBMP primer + DMAHDM) mass fraction of 5%. DMAHDM mass fractions of 7.5% or higher were not used due to a decrease in dentine bond strength when combined with MPC in preliminary study. Similarly, 5% DMAHDM was incorporated into the SBMP-MPC adhesive.
2.3. NACP incorporation into bonding agent
A spray-drying technique was used to synthesize NACP (Ca3[PO4]2) as described previously [40,41]. The NACP had a mean particle size of 116 nm [40,41]. NACP were incorporated into the SBMP adhesive, but not into the primer, because preliminary study showed that adding NACP into primer decreased the dentine bond strength [42,44]. NACP was mixed into the adhesive at NACP/(SBMP adhesive + NACP) = 0%, 20%, 30%, and 40% by mass. NACP mass fraction of less than 20% was not used due to the need for sufficient ion release. NACP of more than 40% was not used due to a decrease in dentine bond strength [42,44].
2.4. Dentine shear bond strength testing
As listed in Table 1, five groups were used for dentine shear bond strength testing. The results showed that group 5 had significantly lower dentine bond strength, while all other groups had dentine bond strengths similar to SBMP control. Therefore, group 5 was not included in subsequent experiments. Groups 1–4 were used in SEM examination, and protein adsorption and biofilm assays.
Table 1.
Compositions of primer and adhesive*
| Group | Primer | Adhesive | Group name |
|---|---|---|---|
| 1 | SBMP primer | SBMP adhesive | SBMP control |
| 2 | SBMP primer + 7.5% MPC + 5% DMAHDM | SBMP adhesive + 7.5% MPC + 5% DMAHDM | 7.5MPC+5DMAHDM |
| 3 | SBMP primer + 7.5% MPC + 5% DMAHDM | SBMP adhesive + 7.5% MPC + 5% DMAHDM + 20% NACP | 7.5MPC+5DMAHDM+20NACP |
| 4 | SBMP primer + 7.5% MPC + 5% DMAHDM | SBMP adhesive + 7.5% MPC + 5% DMAHDM + 30% NACP | 7.5MPC+5DMAHDM+30NACP |
| 5 | SBMP primer + 7.5% MPC + 5% DMAHDM | SBMP adhesive + 7.5% MPC + 5% DMAHDM + 40% NACP | 7.5MPC+5DMAHDM+40NACP |
MPC = 2-methacryloyloxyethyl phosphorylcholine. DMAHDM = dimethylaminohexadecyl methacrylate. NACP = nanoparticles of amorphous calcium phosphate.
Extracted caries-free human molars were used. The tips of the crowns were cut off using a diamond saw (Isomet, Buehler, Lake Bluff, IL). The cut surface of the tooth was ground using 320 grit SiC paper until occlusal enamel was completely removed [27,28]. After etching for 15 s and rinsing with water [45], a primer was applied, and the solvent was removed with air. An adhesive was applied and air-blown for 5 s to produce a thin adhesive layer [15], and then light-cured for 10 s (Optilux-VCL401, Demetron, Danbury, CT). A stainless-steel iris with a central opening (diameter = 4 mm, thickness = 1.5 mm) was held against the adhesive-treated dentine surface. The opening was filled with a composite (TPH, Caulk/Dentsply, Milford, DE) and light-cured for 60 s [45]. The bonded specimens were stored in distilled water at 37 °C for 24 h [27]. The dentine shear bond strength, SD, was measured as previously described [27,45]. A chisel was held parallel to the composite-dentine interface and loaded via a Universal Testing Machine (MTS, Eden Prairie, MN) at 0.5 mm/min until the composite-dentine bond failed. SD = 4P/(πd2), where P is the load at failure, and d is the diameter of the composite [45]. Ten teeth were tested for each group.
2.5. Fabrication of root dentine slabs
Root dentine slabs were fabricated using bovine instead of human root dentine, since the development and inhibition rates of caries are similar in both tissues [46], and bovine teeth are easier to obtain. The specimen preparation procedure followed previous studies [15,16,47]. Extracted bovine incisors with intact roots were used. The root was separated from the crown at 1–2 mm below the cemento-enamel junction using a water-cooled diamond saw (Isomet) [15,16]. Then, root dentine slabs were prepared from buccal and lingual root surfaces with a water-cooled diamond saw [16,47]. The slabs were mounted with sticky wax on plexiglas blocks to facilitate handling, and were serially polished to remove the cementum and flatten the surfaces [16,47]. The polishing procedure was performed with wet SiC papers up to 4000 grit. The polished slabs were examined microscopically (20×), to ensure that a smooth dentine surface was obtained [16,47]. The root slabs were cleaned by sonication for 10 min in distilled water [47]. The final dimensions of the root dentine slabs were approximately 5 mm in length, 3 mm in width, and 2 mm in thickness [47]. One dentine surface of 5 mm × 3 mm faced the cementum, while the other 5 mm × 3 mm surface faced the pulp. Primer and adhesive were applied to the dentine surface that faced the cementum with open dentinal tubules, in order to simulate the clinical application of using an adhesive to cover roots with missing cementum and open tubules [16,47].
2.6. Scanning electron microscopic (SEM) examinations
Because adhesives can provide a barrier to protect the exposed roots from acid attacks [14–17], and a thicker coating is expected to provide a stronger barrier, the coating thickness was measured. Root dentine surface (with cementum removed) was covered with a single coat of an adhesive via the micro-brush supplied in the package, following the manufacturer’s instructions. First, the root dentine slab surface was etched with etchant for 15 s and rinsed with water, and dried with air [48,49]. A primer was applied to the dentine surface and left for 20 s, and air-blown for 5 s [48]. Then, 20 μL of adhesive was applied using pipette and rubbed in for 10 s with the micro-brush [48]. The adhesive was light-cured for 10 s (Optilux). The different viscosity of the four adhesive systems produced different coating thicknesses.
Cross-sectional cutting would yield information on coating thickness and the resin-dentine interface; however, the adhesive coating on dentine could chip and dislodge in the cutting process. To avoid adhesive coating fracture and to enable the measurement of coating thickness, a composite (TPH) was placed on the top of the adhesive coating and light-cured for 60 s, to facilitate the subsequent cutting and the measurement of the adhesive coating thickness [16]. The samples were cut through the center perpendicularly to the coated surface via a diamond saw (Isomet) with copious water. The cross-section was polished with increasingly finer SiC papers up to 4000 grit [15,16]. The specimens were air dried and sputter-coated with gold, and examined in SEM (Quanta 200, FEI, Hillsboro, OR). The thickness of adhesive coating on root dentine was measured for six specimens. Four readings were taken at random locations on each specimen, yielding a total of 24 measurements per group.
To examine the interfaces, additional specimens were treated with 50% phosphoric acid for 30 s and 5% NaOCl for 10 min before being prepared for SEM observation [15]. To observe the adhesive surface texture, root dentine surfaces were coated with one of the adhesives in the same manner as described above (without TPH and without cutting). The surfaces were then air-dried and sputter-coated with gold for SEM observations [15,16].
2.7. Measurement of protein adsorption onto resin surface
To test the protein-repellent properties of groups 1–4, resin disks were fabricated. The cover of a sterile 96-well plate (Costar, Corning Inc., Corning, NY) was used as molds following a previous study [30]. Briefly, 10 μL of a primer was placed in the bottom of each dent of the 96-well plate. After drying with a stream of air, 20 μL of adhesive was applied to the dent and photo-polymerized for 30 s (Optilux), using a Mylar strip covering to obtain a disk of 8 mm in diameter and 0.5 mm in thickness. The cured resin disks were immersed in 200 mL of distilled water and magnetically-stirred with a bar at a speed of 100 rpm for 1 h to remove any uncured monomers, following a previous study [50]. The disks were then sterilized with ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC) and de-gassed for 3 d [30].
The amount of protein adsorbed on resin disks was determined by the micro bicinchoninic acid (BCA) method [51,52]. First, the disks were immersed in phosphate buffered saline (PBS) for 2 h. Then they were immersing in 4.5 g/L bovine serum albumin (BSA) (Sigma-Aldrich) solution at 37 °C for 2 h. This solution had a concentration of 4.5 g of BSA per 1 L of PBS, following previous studies [51,52]. The disks then were rinsed with fresh PBS by stirring at a speed of 300 rpm for 5 min (Bellco Glass, Vineland, NJ), immersed in sodium dodecyl sulfate (SDS) 1 wt % in PBS, and sonicated at room temperature for 20 min to completely detach the BSA adsorbed onto the disk surface. A protein analysis kit (micro BCA protein assay kit, Fisher Scientific, Pittsburgh, PA) was used to determine the BSA concentration in the SDS solution. Briefly, 25 μL of the SDS solution was mixed with 200 μL of the BCA working reagent in a 96-well plate, which was incubated at 60 °C for 30 minutes [51,52]. Then the 96-well plate was cooled down to room temperature and the absorbance at 562 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). Standard curves were prepared using the BSA standard. From the concentration of protein, the amount of protein adsorbed on the disk surface was calculated [51,52]. Six disks were evaluated for each group.
2.8. Saliva collection for dental plaque microcosm biofilm model
The dental plaque microcosm model using human saliva as inoculum has been approved by the University of Maryland Baltimore Institutional Review Board (# HP-00050407). Saliva is ideal for growing dental plaque microcosm biofilms in vitro, with the advantage of maintaining much of the complexity and heterogeneity of the dental plaque in vivo [53]. Saliva was collected from ten healthy adult donors having natural dentition without active caries or periopathology, and without the use of antibiotics within the last 3 months [27,28]. The donors did not brush teeth for 24 h and abstained from food and drink intake for 2 h prior to donating saliva. Stimulated saliva was collected during paraffin chewing and was kept on ice. An equal volume of saliva from each of the ten donors was combined. The saliva was diluted in sterile glycerol to a concentration of 70%, and stored at −80 °C [54].
2.9. Dental plaque microcosm biofilm formation and live/dead assay
The saliva-glycerol stock was added, with 1:50 final dilution, to a growth medium as inoculum [27,28]. The growth medium contained mucin (type II, porcine, gastric) at a concentration of 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L, KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; hemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH 7 [55]. Each resin disk was placed into a 24-well plates, 1.5 mL of inoculum was added to each well, and incubated at 37 °C in 5% CO2 for 8 h. Then, the disks were transferred to new 24-well plates with fresh medium. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for 24 h. This totaled 2 d of incubation, which was shown to form mature biofilms on resin [54].
Disks with biofilms grown for 2 d were rinsed with phosphate buffered saline (PBS) and live/dead stained using the BacLight live/dead bacterial viability kit (Molecular Probes, Eugene, OR) [27,28]. Live bacteria were stained with Syto 9 to produce a green fluorescence. Dead bacteria were stained with propidium iodide to produce a red fluorescence. Disks were examined using an inverted epifluorescence microscope (Eclipse TE2000-S, Nikon, Melville, NY). Six specimens were evaluated for each group. Three randomly chosen fields of view were photographed from each disk, yielding a total of 18 images for each group.
2.10. MTT assay of metabolic activity
Resin disks with 2-day biofilms were transferred to a new 24-well plate for the MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay [27,28]. MTT is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan. One mL of MTT dye (0.5 mg/mL MTT in PBS) was added to each well and incubated at 37 °C in 5% CO2 for 1 h. Metabolically-active bacteria reduced the MTT to purple formazan. Disks were transferred to a new 24-well plate, and 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals. The plate was incubated for 20 min with gentle mixing at room temperature in the dark. Then, 200 μL of the DMSO solution from each well was collected, and its absorbance at 540 nm was measured via a microplate reader (SpectraMax M5). A higher absorbance is related to a higher formazan concentration, which indicates a higher metabolic activity in the biofilm [27,28].
2.11. Lactic acid production and colony-forming unit (CFU) counts of biofilms
Disks with 2-day biofilms were rinsed with cysteine peptone water (CPW) to remove loose bacteria [27]. Disks were transferred to 24-well plates containing buffered peptone water (BPW) plus 0.2% sucrose. The disks were incubated in 5% CO2 at 37 °C for 3 h to allow biofilms to produce acid [27]. The BPW solutions were collected for lactate analysis using an enzymatic method. The 340-nm absorbance was measured with the microplate reader. Standard curves were prepared using a standard lactic acid (Supelco, Bellefonte, PA).
Disks with biofilms were transferred into tubes with 2 mL CPW, and biofilms were harvested by sonication and vortexing (Fisher, Pittsburgh, PA) [27,28]. Three types of agar plates were prepared. First, tryptic soy blood agar culture plates were used to determine total microorganisms [55]. Second, mitis salivarius agar (MSA) plates with 15% sucrose were used to determine total streptococci [56]. This is because MSA contains selective agents including crystal violet, potassium tellurite and trypan blue, which inhibit most Gram-negative bacilli and most Gram-positive bacteria except streptococci, thus enabling the streptococci to grow [56]. Third, cariogenic mutans streptococci are known to be resistant to bacitracin, and this property is used to isolate mutans streptococci from the highly heterogeneous oral microflora [55]. Therefore, MSA agar culture plates plus 0.2 units of bacitracin per mL was used to determine mutans streptococci [55]. The bacterial suspensions were serially diluted and spread onto agar plates for CFU analysis.
2.12. Statistical analysis
All data were first checked for normal distribution with the Kolmogorov-Smirnov test. One-way and two-way analyses of variance (ANOVA) were performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used to compare the data at a p value of 0.05.
3. Results
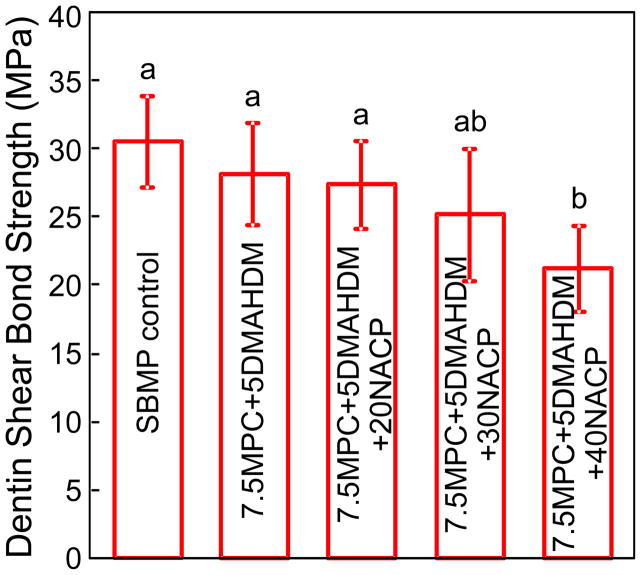
Fig. 1 plots the dentine shear bond strengths (mean ± sd; n = 10). Groups 1–4 had similar dentine bond strengths (p > 0.1). Group 5 had a lower strength than SBMP control (p < 0.05). Therefore, incorporating 7.5% MPC, 5% DMAHDM, and NACP up to 30% into SBMP did not significantly compromise the dentine bond strength.
Fig. 1.
Dentine shear bond strengths (mean ± sd; n = 10). All the strengths were not significantly different from each other, except the 7.5MPC+5DMAHDM+40NACP group. Dissimilar letters indicate values that are significantly different from each other (p < 0.05).
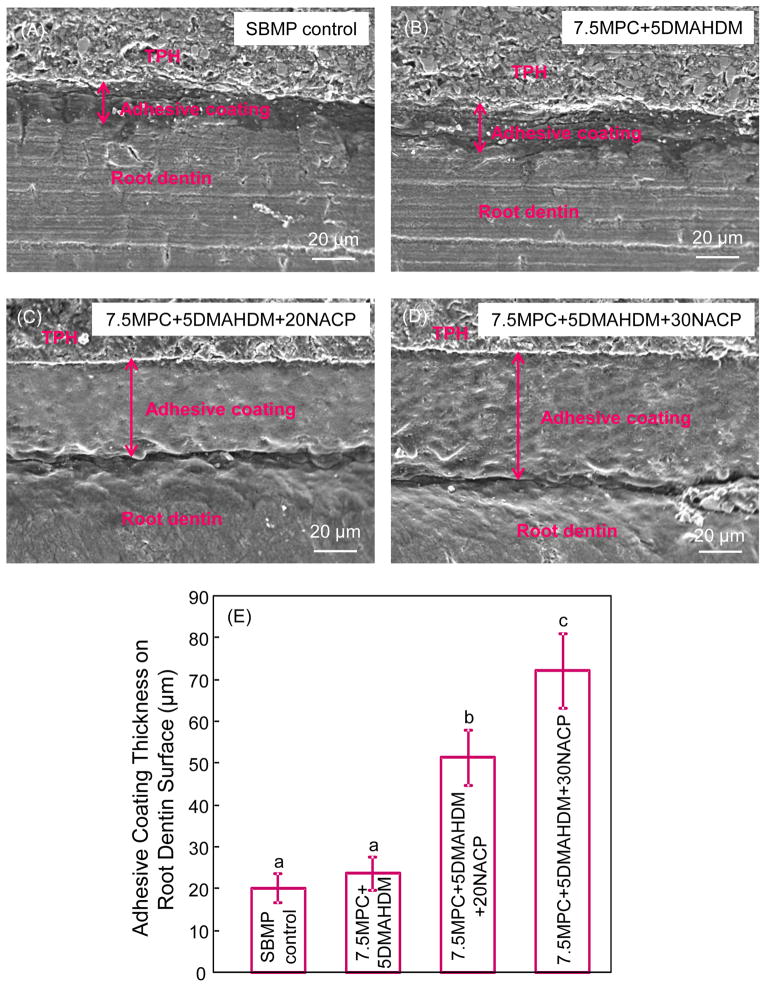
Typical SEM images of adhesive coating thickness on root dentine were shown in Fig. 2 (A–D). The mean values of coating thickness are plotted in Fig. 2 (E) (mean ± sd; n = 6). Adding NACP greatly increased the adhesive coating thickness. The coating thickness of 7.5MPC+5 DMAHDM+30NACP group was greater than other groups (p < 0.05).
Fig. 2.
Typical SEM images showing adhesive coating layers on tooth root dentine. TPH is a composite (Dentsply), used to facilitate the cutting of cross-sections and avoid adhesive coating layer chipping/fracture during the cutting process. The mean value of adhesive coating thickness for each adhesive is plotted in (E) (mean ± sd; n = 6). Dissimilar letters indicate values that are significantly different from each other (p < 0.05).
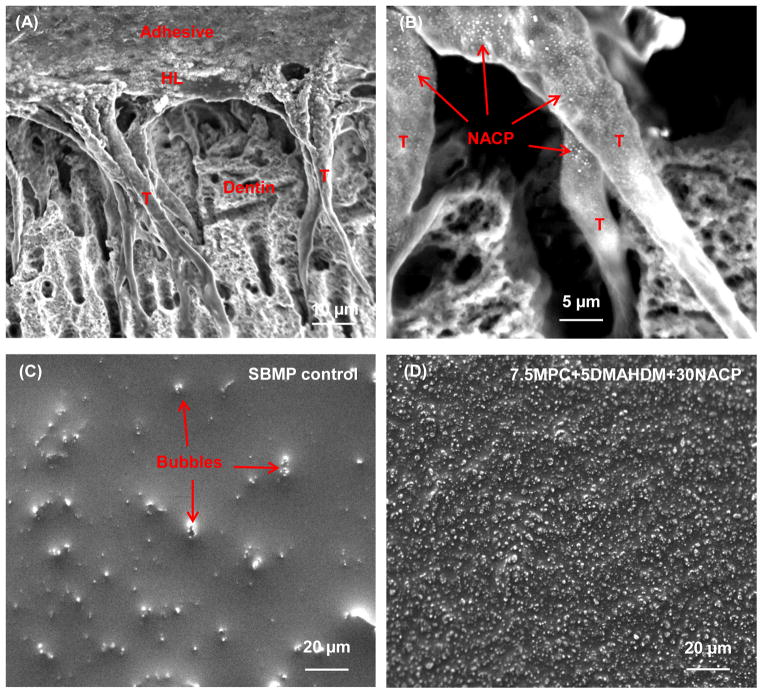
Fig. 3 shows typical SEM images of (A, B) dentine-adhesive interfaces, and (C, D) surface texture of adhesive coating on root dentine. The example shown in (A) was for the 7.5MPC+5DMAHDM+30NACP group. Numerous resin tags “T” from well-filled dentinal tubules were visible in (A). “HL” refers to the hybrid layer between the adhesive and the underlying mineralized dentine. Arrows in (B) indicate examples of NACP in dentinal tubules. In (C), some bubble-shaped deficiencies were observed on the cured SBMP control adhesive. In contrast, in (D), the 7.5MPC+5DMAHDM+30NACP group showed uniform surfaces and the exposed dentine was hermetically sealed with resinous material.
Fig. 3.
Representative SEM images of dentine-adhesive interfaces at the cross-sections, as well as adhesive coating surface textures (without cross-section). (A, B) Cross-section for 7.5MPC+5 DMAHDM+30 NACP group at a lower and higher magnification. The adhesive filled the dentinal tubules and formed resin tags “T”. “HL” indicates the hybrid layer between the adhesive and the underlying mineralized dentine. Arrows in (B) indicate NACP in the dentinal tubules. (C) Surface of SBMP control coating on root dentine, and (D) surface of 7.5MPC+5DMAHDM+30NACP coating on root dentine. In (C), arrows indicate air bubbles in control adhesive coating. In (D), the 7.5MPC+5DMAHDM+30NACP adhesive coating had a solid and dense appearance without air bubbles. The adhesive had completely sealed the root dentine surface.
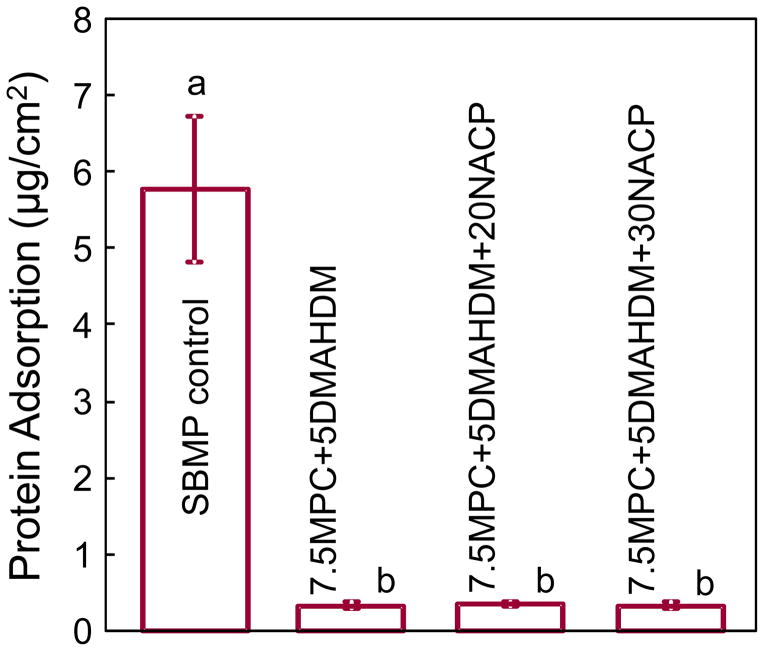
Protein adsorption onto resin surfaces is plotted in Fig. 4 (mean ± sd; n = 6). Incorporating MPC into the adhesive significantly decreased the amount of protein adsorption. The SBMP control had the highest amount of protein adsorption, which was nearly 18-fold higher than the other groups containing MPC (p < 0.05).
Fig. 4.
The amount of protein adsorption onto resin surfaces for each group (mean ± sd; n = 6). SBMP control had the most protein adsorption, which was nearly 18-fold higher than the other groups containing protein-repellent agent MPC (p < 0.05).
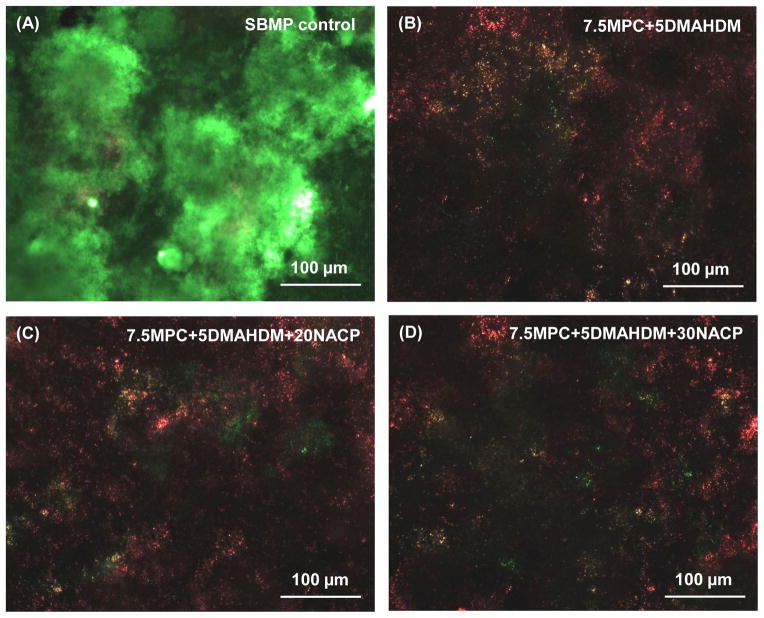
Typical live/dead images of 2-day biofilms on resin are shown in Fig. 5. SBMP control was fully covered by primarily live bacteria. In contrast, (B), (C) and (D) showed noticeably less bacterial adhesion, and the biofilms consisted of primarily dead bacteria.
Fig. 5.
Representative live/dead staining images of biofilms adherent on resin disks. The live bacteria were stained green, and the dead bacteria were stained red. When live and dead bacteria were in close proximity or on the top of each other, the staining had yellow/orange colors. SBMP control was fully covered by primarily live bacteria. In contrast, (B), (C) and (D) showed noticeably less bacterial adhesion, and the biofilms consisted of primarily dead bacteria.
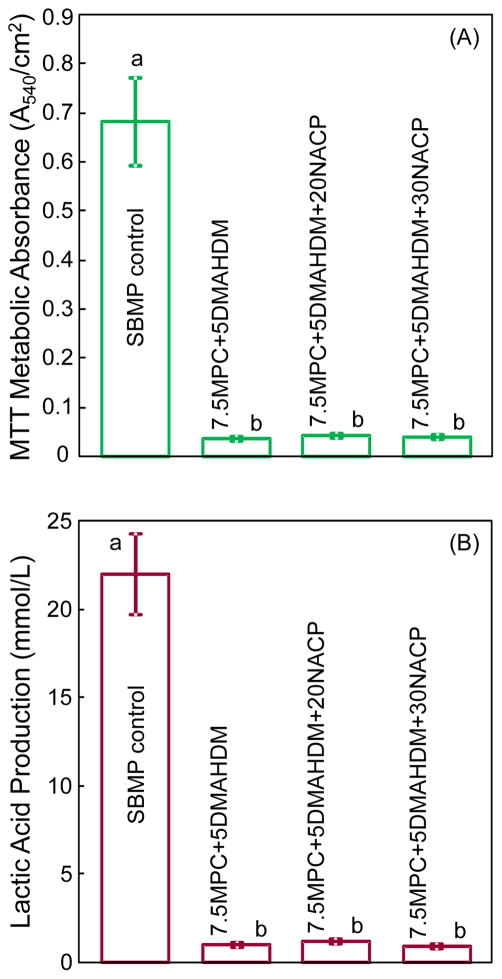
Quantitative viability of the 2-day biofilms on resin disks is shown in Fig. 6: (A) metabolic activity, and (B) lactic acid production (mean ± sd; n = 6). Biofilms on SBMP control had the highest metabolic activity and the most lactic acid production. The MPC-DMAHDM-NACP containing resin had much less metabolic activity and lactic acid production of biofilms, compared to SBMP control (p < 0.05).
Fig. 6.
Biofilm viability on resin disks: (A) metabolic activity, and (B) lactic acid production (mean ± sd; n = 6). Biofilms on the adhesive containing MPC-DMAHDM-NACP had metabolic activity that was about 1/20 that on SBMP control, and lactic acid production about 1/25 that on SBMP control.
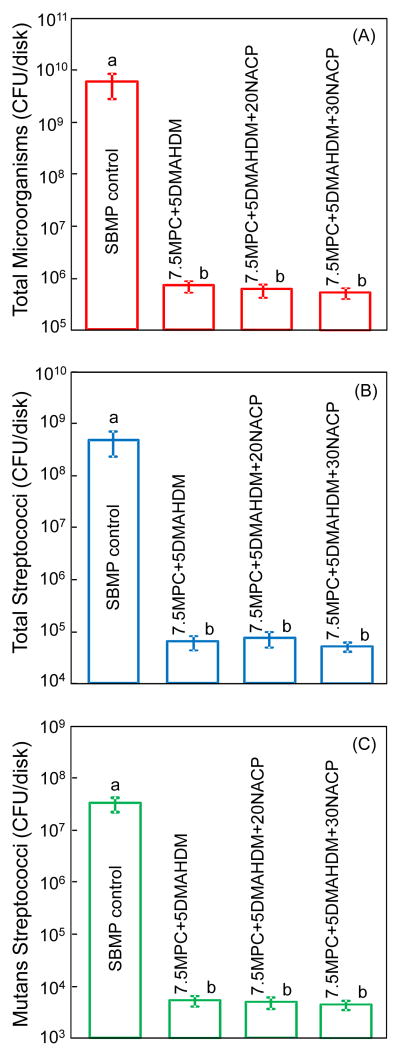
Fig. 7 plots the 2-day biofilm CFU for: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). Note the log scale in y axis. All three CFU counts on the MPC-DMAHDM-NACP containing resin were more than 4 orders of magnitude lower than SBMP control (p < 0.05).
Fig. 7.
Colony-forming unit (CFU) counts for: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). All three CFU counts on the adhesive containing MPC-DMAHDM-NACP were more than 4 orders of magnitude lower than those on SBMP control (p < 0.05).
4. Discussion
In the present study, a multifunctional adhesive system was developed by combining MPC for protein-repellent ability, DMAHDM for antibacterial potency, and NACP for remineralization for the first time. The 7.5MPC+5DMAHDM+30NACP adhesive resin created a hermetic sealing of tooth root dentine surface with a relatively thick resinous layer. This coating may act as a physical barrier to protect tooth root surface after losing cementum, and for seniors with gingival recession and root exposure. The new adhesive resin with 7.5MPC+5DMAHDM+30NACP greatly reduced protein adsorption, bacteria attachment and biofilm growth, metabolic activity, CFU counts, and lactic acid production. These benefits were achieved without compromising the dentine shear bond strength. Therefore, this multifunctional adhesive system is promising to prevent root caries when used to cover roots, and to inhibit secondary caries when used to bond restorations in tooth cavities.
Cementum is the first target for plaque bacteria to attack when the root surface is exposed to the oral environment. The thin cementum can be easily removed by intensive root planing during the treatment of periodontal diseases or by over-zealous tooth-brushing [36]. The vulnerability of the cementum causes the underlying dentine to be prone to exposure, hence increasing the risk of dentine hypersensitivity and root dentine caries formation [38]. For this reason, root dentine slabs without cementum were used in the present study to test the adhesive as a barrier coating on root dentine. It was reported that a barrier film coating plays an important role in protecting dentine from physical, chemical and biological stimuli in the prepared dentine [15,16]. Therefore, such a protective coating may have the potential to cover exposed root dentinal surfaces, eliminate sensitivity, and prevent caries formation. However, it was reported that coating with adhesives was unable to provide complete and long-term prevention for the demineralization induced by acid attack [14,16,57]. One reason for this phenomenon may be that the coating thickness of the adhesives was too thin to act as a substantive physical barrier against demineralization [14,16,57], especially after clinical polishing and daily tooth-brushing [14]. The different thicknesses of the coating is related to the viscosity and application procedures [15,16]. A previous study reported that mixing filler particles into the adhesive could increase the viscosity and hence the thickness of the coating [16]. Another study indicated that adding NACP could increase the viscosity of the adhesive [42]. In the present study, the four adhesives were applied onto root dentine in the same manner according to SBMP manufacturer’s instructions. Previous studies reported that a coating thickness of about 60 μm effectively prevented demineralization by acid attack to root dentine [15,16]. A single coat of SBMP control created a thin coating of about 20 μm (Fig. 2), which might be too thin as a protective layer. Triple-coats of SBMP control may create enough coating thickness as reported by previous studies [15,16]. However, the coating needs to be light-cured after each coating [16,57]; three coats and three light-curing would consume significant time for the dentist. In addition, due to the low viscosity of unfilled adhesive, if too much of adhesive was applied onto the exposed root surface, the excess adhesive would flow away to undesired places such as gingiva and mucosa, thus leading to contamination. In contrast, incorporating NACP into the adhesive increased the viscosity of adhesive, thereby greatly increasing the coating thickness. A single coat of 7.5MPC+5DMAHDM+30NACP paste created a coating thickness of nearly 70 μm (Fig. 2), 3-fold that of SBMP control. Such a coating thickness was consistent with previous studies showing that this coating thickness would be effective to serve as a protective barrier for root dentine [15,16].
Covering the exposed root dentine and sealing dentinal tubules can reduce hypersensitivity and inhibit root caries [15,16]. The surface texture of the adhesive-coated root dentine slab was observed by SEM. The SBMP control coating on root dentine showed a porous appearance with air bubbles (Fig. 3C). In contrast, a dense and solid coating was produced by the 7.5MPC+5DMAHDM+30NACP group. Furthermore, penetration of the adhesive resin into the etched dentine is required to producing an impermeable coating [15]. Indeed, numerous resin tags were achieved in the 7.5MPC+5DMAHDM+30NACP group. The hybrid layer between the adhesive and the underlying mineralized dentine was formed via bonding agent infiltrating into the demineralized collagen layer [42]. Accordingly, the adhesive containing MPC-DMAHDM-NACP not only produced a coating thickness on root dentine known to be an effective protective barrier, but also formed an integrated hybrid layer with dentine for mechanical interlock.
Besides serving as a physical barrier coating, there are four other advantages of this novel multifunctional adhesive. First, hydrophilic material surfaces are usually more resistant to protein adsorption and bacterial adhesion than hydrophobic ones [58]. The MPC polymer is highly hydrophilic [31,32]. In the hydrated MPC polymer, there is an abundance of free water but no bound water [32]. The presence of bound water would cause protein adsorption [32,59]. In contrast, the large amount of free water around the phosphorylcholine group is considered to detach proteins effectively, thereby repelling protein adsorption [32,59]. In the present study, incorporating MPC into the adhesive resin substantially decreased protein adsorption (Fig. 4), thereby reducing bacterial attachment. It should be noted that surface roughness of the specimens would interfere with protein adsorption measurement. In the present study, mylar strips were used to cover the specimen surfaces, which produced smooth surfaces with relatively uniform and very small roughness among all the groups. After photo-polymerization, the mylar strip was removed and no further grinding or polishing was done to the resin surface. The resin surfaces were examined in microscope to be free of significant porosity, except occasional microporosity and nanoporosity which were similar among all the groups.
Second, QAMs had bacteriolysis effects, because their positively-charged quaternary amine N+ can attract the negatively-charged cell membrane of bacteria, which could disrupt the cell membrane and cause cytoplasmic leakage [60]. A previous study prepared antimicrobial polymeric brushes and showed that high density cationic surfaces effectively killed bacteria, and long cationic polymers can penetrate bacterial cells to disrupt membranes, like a needle bursting a balloon [61]. This is consistent with more recent studies on a series of new QAMs with chain lengths varying from 3 to 18 [29,30]. DMAHDM with a chain length of 16 had the strongest antibacterial activity, and the antibacterial potency increased with increasing quaternary amine charge density on the adhesive resin [29,30]. It should be noted that while the 2-day biofilms on SBMP in Fig. 5A were primarily alive with few dead bacterial colonies, there were some dead colonies near the bottom of the biofilm due to less nutrition than those at the top of the biofilm. A recent study showed that there were nearly 100% live bacteria near the top of the three-dimensional biofilm on SBMP control, but the live bacteria fraction decreased to approximately 65% at the bottom layer of the biofilm [30]. In contrast, the SBMP containing DMAHDM had mainly dead bacteria throughout the entire thickness of the biofilm [30].
Third, a potential limitation of QAMs is that the deposit of salivary proteins on surfaces could decrease the efficacy of “contact-inhibition”, thus reducing the antibacterial potency [60]. A previous study demonstrated that a saliva-derived protein film on the cationic antibacterial surfaces reduced the original bactericidal effect [62]. Because the MPC-containing resin can greatly reduce the protein adsorption, there would be much less protein on the resin and hence more direct resin-bacteria contact, thereby enhancing the antibacterial effect of DMAHDM. Indeed, the results in Figs. 5 to 7 confirmed that combining MPC with DMAHDM greatly reduced the biofilm viability, metabolic activity, CFU and lactic acid production.
Forth, the MPC-DMAHDM-NACP containing adhesive is not only antibacterial, but also has Ca and P ion release and remineralization capabilities. Several recent studies evaluated the ion release and remineralization properties of NACP nanocomposites [40–44]. The NACP nanocomposite was “smart” and increased the Ca and P ion release at a cariogenic pH of 4, when these ions would be most needed to inhibit caries [40,41]. A previous study showed that a composite with 30% NACP neutralized an acid attack, and raised the solution pH from a cariogenic pH of 4 to a safe pH of 6.5 [41]. Therefore, incorporating 20% or 30% NACP into adhesive is expected to provide acid neutralization and remineralization benefits.
Besides coating tooth root surfaces to prevent root caries, this novel multifunctional adhesive system can potentially also be used to bond a restoration in tooth cavity [23,24]. This is because secondary caries at the tooth-restoration margins is a primary reason for restoration failure [4,5]. The adhesive with MPC, DMAHDM, and NACP achieved protein-repellent, antibacterial, and remineralization capabilities without compromising the dentine bond strength. By inhibiting the formation of biofilms which produce acids and enzymes to cause tooth decay [5,9], the adhesive containing MPC, DMAHDM and NACP has the potential to inhibit secondary caries. When a tooth decay is removed, it is usually impossible to completely remove all the bacteria in the cavity. There are often residual bacteria left in the prepared tooth cavity, sometimes due to the purpose of preserving more tooth structure or avoiding pulp perforation [25]. Therefore, more carious tissues with residual bacteria could be left in the prepared tooth cavity. Uncured primer with MPC and DMADDM has direct contact with the tooth structure and can flow into dentinal tubules to kill the residual bacteria. During service, there are also new invading bacteria because of microgaps at the tooth-restoration interfaces [19,22]. It is beneficial to use the MPC-DMAHDM-NACP containing adhesive to inhibit bacterial invasion and remineralize lesions. The novel method of incorporating a protein-repellent agent (MPC), an antibacterial agent (DMAHDM), and remineralizing agent (NACP) together in the same adhesive may have wide applicability to other bonding systems, sealants and cements. It should be noted that this is an in vitro study without the complications of the oral environment such as saliva flow, food debris and wear in vivo. Caution should be used in extending the in vitro benefits to patients in vivo. For example, the charged moieties of quaternary ammonium methacrylates could potentially promote hydration of the polymer leading to swelling and degradation. In addition, the hermetic sealing of the interface between the adhesive and dentin was not measured in this investigation. Further studies are needed to investigate these issues as well as the long-term durability of this multifunctional adhesive system under in vivo conditions.
5. Conclusions
The present study developed a multifunctional adhesive for prevention of root caries and secondary caries by combining MPC for protein-repellent ability, DMAHDM for antibacterial potency and NACP for remineralization for the first time. The adhesive containing MPC-DMAHDM-NACP sealed root dentine with a relatively thick resinous coating as a physical barrier to protect root surface. The adhesive greatly reduced protein adsorption, bacterial attachment, biofilm viability, metabolic activity, CFU counts, and lactic acid production, without compromising dentine bond strength. Therefore, this novel adhesive is promising for coating tooth roots where cementum is missing and for seniors with gingival recession and root exposure, to inhibit root caries and secondary caries. The method of incorporating triple agents (MPC as a protein-repellent agent, DMAHDM as an antibacterial agent, and NACP as a remineralizing agent) may be applicable to a wide range of dental bonding agents, cements, sealants and composites for anti-caries capability.
Highlights.
A novel adhesive for prevention of tooth root caries and secondary caries was developed containing a protein-repellent agent, an antibacterial monomer, and remineralization nanoparticles for the first time. A single coat of this adhesive on tooth root dentine produced a coating thickness of approximately 70 μm and completely sealed all dentinal tubules to provide a barrier against biofilm acids. In addition, this adhesive resin had a 95% reduction in protein adsorption, was strongly antibacterial, and reduced biofilm colony-forming units by four orders of magnitude. Therefore, this novel multifunctional adhesive with strong protein-repellent, antibacterial and remineralization properties is promising to coat tooth roots to prevent root caries, as well as to bond restorations in tooth cavities to inhibit secondary caries. The combined use of triple agents (protein-repellent agent, antibacterial agent, and remineralization agent) may have wide applicability in bonding agents, cements, sealants and composites to inhibit caries.
Acknowledgments
We thank Drs. Junling Wu and Ling Zhang for discussions and experimental help. This study was financially supported by the National Natural Science Foundation of China (NSFC grant No. 81271184) (NZ), the Beijing Municipal Science and Technology Commission, China (Grant No. Z121107001012008) (NZ), NIH R01 DE17974 (HX), and a Seed Grant (HX) from the University of Maryland School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griffin SO, Griffin PM, Swann JL, Zlobin N. Estimating rates of new root caries in older adults. Journal of Dental Research. 2004;83:634–8. doi: 10.1177/154405910408300810. [DOI] [PubMed] [Google Scholar]
- 2.Banting DW, Papas A, Clark DC, Proskin HM, Schultz M, Perry R. The effectiveness of 10% chlorhexidine varnish treatment on dental caries incidence in adults with dry mouth. Gerodontology. 2000;17:67–76. doi: 10.1111/j.1741-2358.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 3.Curzon MEJ, Preston AJ. Risk groups: nursing bottle caries/caries in the elderly. Caries Research. 2004;38:24–33. doi: 10.1159/000074359. [DOI] [PubMed] [Google Scholar]
- 4.Ferracane JL. Resin composite – state of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Ferracane JL. Resin-based composite performance: are there some things we can’t predict? Dental Materials. 2013;29:51–8. doi: 10.1016/j.dental.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 7.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Reports. 2007;122:657–63. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banting DW. The diagnosis of root caries. Journal of Dental Education. 2001;65:991–6. [PubMed] [Google Scholar]
- 9.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. Journal of Dental Research. 2010;89:657–65. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 10.Davies RM. The rational use of oral care products in the elderly. Clinical Oral Investigations. 2004;8:2–5. doi: 10.1007/s00784-003-0234-3. [DOI] [PubMed] [Google Scholar]
- 11.Ravald N, Birkhed D. Prediction of root caries in periodontally treated patients maintained with different fluoride programmes. Caries Research. 1992;26:450–8. doi: 10.1159/000261486. [DOI] [PubMed] [Google Scholar]
- 12.Slot DE, Vaandrager NC, Van Loveren C, Van Palenstein Helderman WH, Van der Weijden GA. The effect of chlorhexidine varnish on root caries: a systematic review. Caries Research. 2011;45:162–73. doi: 10.1159/000327374. [DOI] [PubMed] [Google Scholar]
- 13.Niessen LC. Chlorhexidine varnish, sodium fluoride varnish, and silver diamine fluoride solution can prevent the development of new root caries in elders living in senior homes in Hong Kong. Journal of Evidence-based Dental Practice. 2012;12:95–6. doi: 10.1016/j.jebdp.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Gando I, Ariyoshi M, Ikeda M, Sadr A, Nikaido T, Tagami J. Resistance of dentin coating materials against abrasion by toothbrush. Dental Materials Journal. 2013;32:68–74. doi: 10.4012/dmj.2012-186. [DOI] [PubMed] [Google Scholar]
- 15.Kaneshiro AV, Imazato S, Ebisu S, Tanaka S, Tanaka Y, Sano H. Effects of a self-etching resin coating system to prevent demineralization of root surfaces. Dental Materials. 2008;24:1420–7. doi: 10.1016/j.dental.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Daneshmehr L, Matin K, Nikaido T, Tagami J. Effects of root dentin surface coating with all-in-one adhesive materials on biofilm adherence. Journal of Dentistry. 2008;36:33–41. doi: 10.1016/j.jdent.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Ma S, Imazato S, Chen JH, Mayanagi G, Takahashi N, Ishimoto T, et al. Effects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dental Materials Journal. 2012;31:909–15. doi: 10.4012/dmj.2012-061. [DOI] [PubMed] [Google Scholar]
- 18.El-Safty S, Akhtar R, Silikas N, Watts DC. Nanomechanical properties of dental resin-composites. Dental Materials. 2012;28:1292–300. doi: 10.1016/j.dental.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Watts DC, Alnazzawi A. Temperature-dependent polymerization shrinkage stress kinetics of resin-composites. Dental Materials. 2014;30:654–60. doi: 10.1016/j.dental.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dental Materials. 2002;18:436–44. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 21.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues FP, Lima RG, Muench A, Watts DC, Ballester RY. A method for calculating the compliance of bonded-interfaces under shrinkage: Validation for Class I cavities. Dental Materials. 2014;30:936–44. doi: 10.1016/j.dental.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J. State of the art of self-etch adhesives. Dental Materials. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37:289–96. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Weir MD, Chen J, Xu HH. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dental Materials. 2014;30:433–41. doi: 10.1016/j.dental.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H, Weir MD, Antonucci JM, Schumacher GE, Zhou XD, Xu HHK. Three-dimensional biofilm evaluation on bonding agents containing quaternary ammonium methacrylates with different chain lengths. International Journal of Oral Sciences. 2014;6:77–86. doi: 10.1038/ijos.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishihara K, Ueda T, Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polymer Journal. 1990;22:355–60. [Google Scholar]
- 32.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? Journal of Biomedical Materials Research. 1998;39:323–30. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 33.Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria material interactions. European Cells and Materials. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Melo MA, Bai Y, Xu HH. Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine. Journal of Dentistry. 2014;42:1284–91. doi: 10.1016/j.jdent.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Ma J, Melo MA, Weir MD, Bai Y, Xu HH. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. Journal of Dentistry. 2015;43:225–34. doi: 10.1016/j.jdent.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritz L, Hefti AF, Rateitschak KH. An in vitro investigation on the loss of root substance in scaling with various instruments. Journal of Clinical Periodontology. 1991;18:643–7. doi: 10.1111/j.1600-051x.1991.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoppenbrouwers PM, Driessens FC, Borggreven JM. The mineral solubility of human tooth roots. Archives of Oral Biology. 1987;32:319–22. doi: 10.1016/0003-9969(87)90085-9. [DOI] [PubMed] [Google Scholar]
- 38.Keltjens H, Shaeken T, van der Hoeven H. Preventive aspects of root caries. International Dental Journal. 1993;43:143–8. [PubMed] [Google Scholar]
- 39.Langhorst SE, O’Donnell JNR, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dental Materials. 2009;25:884–91. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dental Materials. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weir MD, Chow LC, Xu HHK. Remineralization of demineralized enamel via calcium phosphate nanocomposite. Journal of Dental Research. 2012;91:979–984. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dental Materials. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imazato S, Ma S, Chen JH, Xu HH. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dental Materials. 2014;30:97–104. doi: 10.1016/j.dental.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melo MAS, Weir MD, Rodrigues LKA, Xu HH. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dental Materials. 2013;29:231–40. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonucci JM, O’Donnell JN, Schumacher GE, Skrtic D. Amorphous calcium phosphate composites and their effect on composite-adhesive-dentin bonding. Journal of Adhesion Science and Technology. 2009;23:1133–47. doi: 10.1163/156856109x432767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hara AT, Queiroz CS, Paes Leme AF, Serra MC, Cury JA. Caries progression and inhibition in human and bovine root dentine in situ. Caries Research. 2003;37:339–44. doi: 10.1159/000072165. [DOI] [PubMed] [Google Scholar]
- 47.Diamanti I, Koletsi-Kounari H, Mamai-Homata E, Vougiouklakis G. In vitro evaluation of fluoride and calcium sodium phosphosilicate toothpastes, on root dentine caries lesions. Journal of Dentistry. 2011;39:619–28. doi: 10.1016/j.jdent.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Cheng L, Zhang K, Weir MD, Liu H, Zhou X, Xu HH. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dental Materials. 2013;29:462–72. doi: 10.1016/j.dental.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amaral NG, Rezende ML, Hirata F, Rodrigues MG, Sant’ana AC, Greghi SL. Comparison among four commonly used demineralizing agents for root conditioning: a scanning electron microscopy. Journal of Applied Oral Science. 2011;19:469–75. doi: 10.1590/S1678-77572011000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 51.Moro T, Kawaguchi H, Ishihara K, Kyomoto M, Karita T, Ito H. Wear resistance of artificial hip joints with poly(2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials. 2009;30:2995–3001. doi: 10.1016/j.biomaterials.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 52.Sibarani J, Takai M, Ishihara K. Surface modification on microfluidic devices with 2-methacryloyloxyethyl phosphorylcholine polymers for reducing unfavorable protein adsorption. Colloids and Surfaces B: Biointerfaces. 2007;54:88–93. doi: 10.1016/j.colsurfb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 53.McBain AJ. In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 54.Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM. Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Research. 2011;45:87–92. doi: 10.1159/000324084. [DOI] [PubMed] [Google Scholar]
- 55.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. Development and characterization of a simple perfused oral microcosm. Journal of Applied Microbiology. 2005;98:624–34. doi: 10.1111/j.1365-2672.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- 56.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre Dos Santos M, et al. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. European Journal of Oral Sciences. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 57.Kuramoto A, Imazato S, Walls AW, Ebisu S. Inhibition of root caries progression by an antibacterial adhesive. Journal of Dental Research. 2005;84:89–93. doi: 10.1177/154405910508400116. [DOI] [PubMed] [Google Scholar]
- 58.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Journal of Biomedical Materials Research. 1998;43:338–48. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 59.Kyomoto M, Moro T, Miyaji F, Hashimoto M, Kawaguchi H, Takatori Y, et al. Effects of mobility/immobility of surface modification by 2-methacryloyloxyethyl phosphorylcholine polymer on the durability of polyethylene for artificial joints. Journal of Biomedical Materials Research Part A. 2009;90:362–71. doi: 10.1002/jbm.a.32092. [DOI] [PubMed] [Google Scholar]
- 60.Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, et al. Antibacterial effect of bactericide immobilized in resin matrix. Dental Materials. 2009;25:424–30. doi: 10.1016/j.dental.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surfaces-2: how high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–9. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Müller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, et al. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]