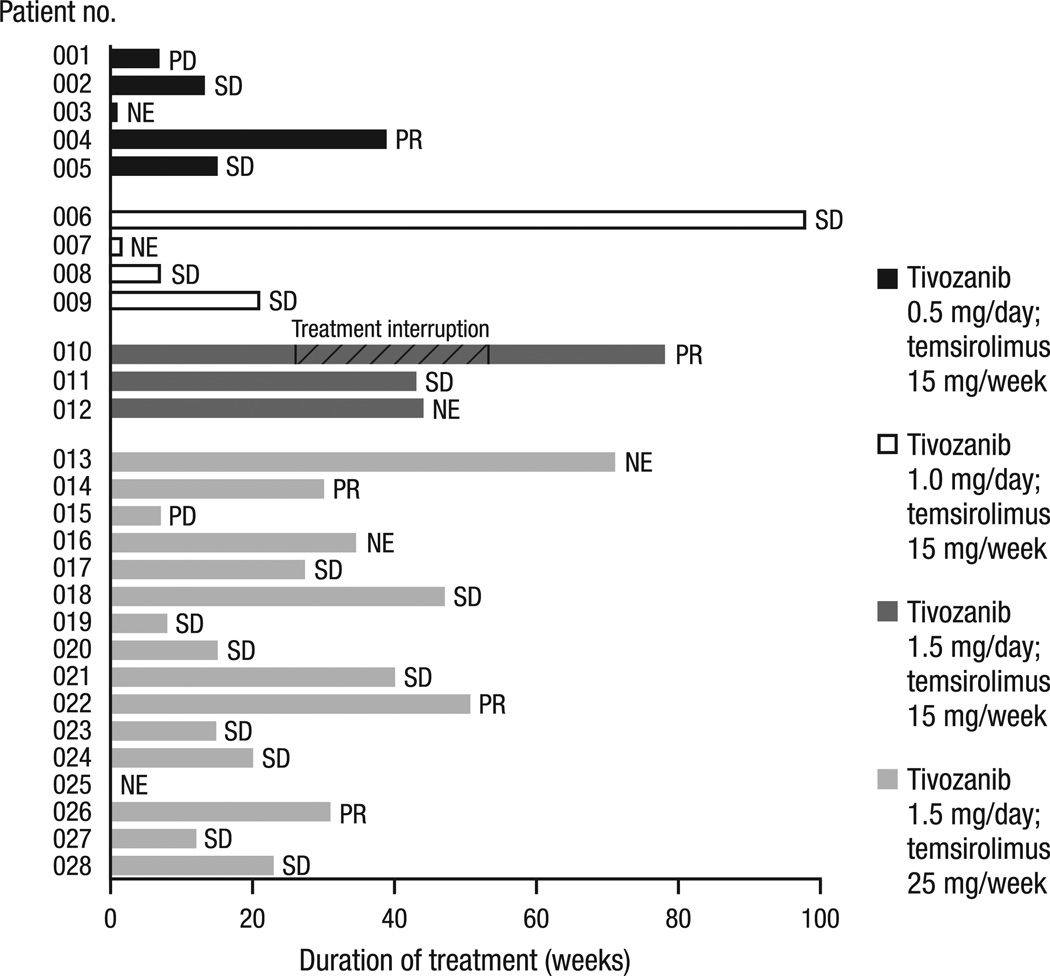
Fig. 2.
Duration of treatment and best response. Response was determined for the 22 patients evaluable for response: five (23%) patients achieved a best response of PR, and 15 (68%) patients achieved a best response of SD. Patient 010 received six cycles of study treatment before the patient’s treatment was interrupted (September 2009) when a suspected abscess appeared in the peripheral lung. He had antimicrobial treatment and resection, showing no malignancy in that area. The mediastinal lymph nodes that were the site of the metastatic RCC were about the same (SD) after the treatment hiatus, and he restarted on treatment (March 2010) following IRB approval. The patient continued study treatment for another 6 months before rolling over to a long-term treatment study. Of the six patients who were NE for response, one patient withdrew prior to tivozanib administration, two patients received <2 cycles of tivozanib before withdrawing for reasons other than progressive disease, and it was later determined that three others did not satisfy the entry criteria (no histologically confirmed RCC with clear cell component; received herbal preparations/supplements within 2 weeks prior to or during the study; and ongoing hemoptysis/clinically significant bleeding and experimental therapy within 4 weeks prior to and during the study). PD, progressive disease; SD, stable disease; NE, not evaluable; PR, partial response; RCC, renal cell cancer; IRB, Institutional Review Board.