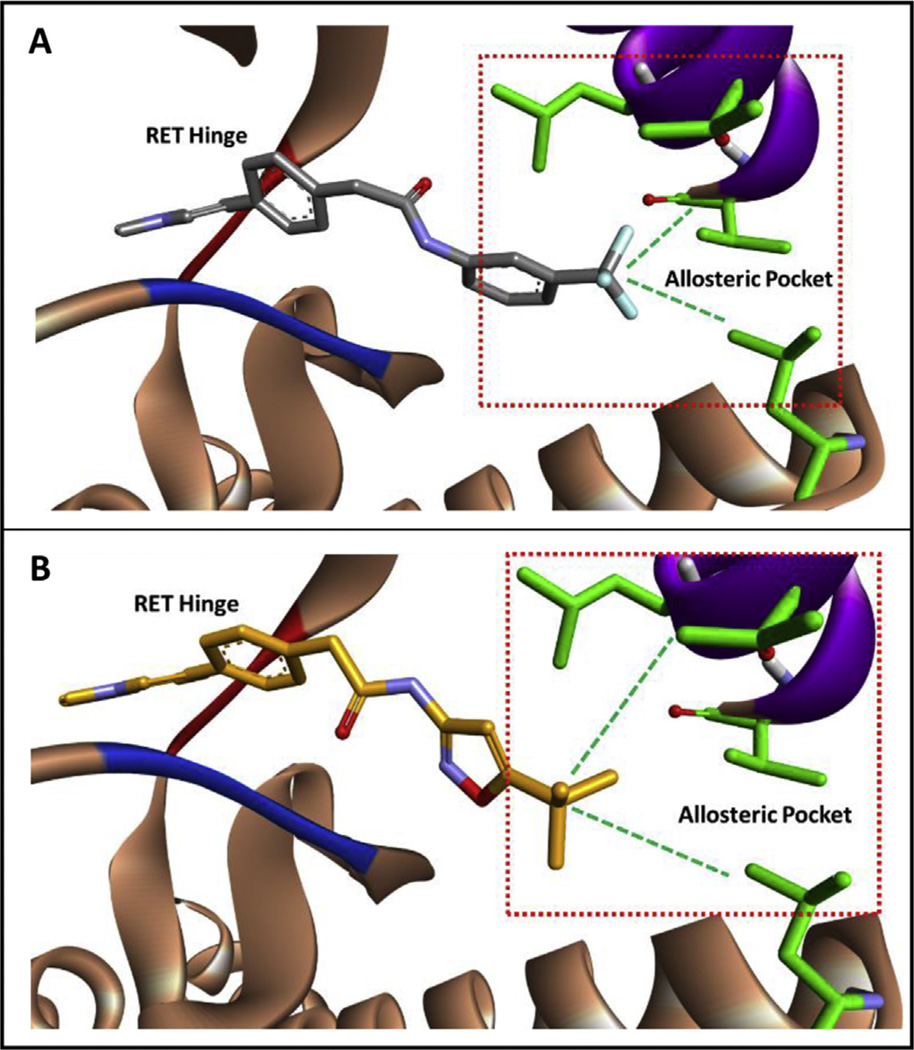
Fig. 2.
A) Computational modeling of 6f in the RET kinase. The –CF3 moiety engages hydrophobic regions at the back of the allosteric pocket. B) Computation modeling of 6g in the RET kinase. The tert-butyl group is more bulky than the –CF3 moiety and is predicted to better engage hydrophobic regions at the back of the RET allosteric pocket. The RET hinge is shown in red, the DFG loop is shown in blue, and the c-Helix is shown in purple. Computational experiments were completed with AutoDock Vina [39]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)