Abstract
Theory of mind (ToM) is the ability to interpret and understand human behaviour by representing the mental states of others. Like many human capacities, ToM is thought to develop through both complex biological and socialization mechanisms. However, no study has examined the joint effect of genetic and environmental influences on ToM. This study examined how variability in the oxytocin receptor gene (OXTR) and parenting behaviour—two widely studied factors in ToM development—interacted to predict ToM in pre-school-aged children. Participants were 301 children who were part of an ongoing longitudinal birth cohort study. ToM was assessed at age 4.5 using a previously validated scale. Parenting was assessed through observations of mothers’ cognitively sensitive behaviours. Using a family-based association design, it was suggestive that a particular variant (rs11131149) interacted with maternal cognitive sensitivity on children’s ToM (P = 0.019). More copies of the major allele were associated with higher ToM as a function of increasing cognitive sensitivity. A sizeable 26% of the variability in ToM was accounted for by this interaction. This study provides the first empirical evidence of gene–environment interactions on ToM, supporting the notion that genetic factors may be modulated by potent environmental influences early in development.
Keywords: theory of mind, parenting, cognitive sensitivity, oxytocin receptor gene, gene–environment interaction
Theory of mind (ToM) is the social cognitive ability to represent the mental states of others in order to interpret human behaviour—i.e. we reason and make sense about others’ actions by imputing their underlying desires, intentions, emotions and beliefs. ToM is critical for human social interaction, the ability to form meaningful interpersonal relationships, and overall psychosocial health and well-being (Baron-Cohen et al., 2013). Indeed, numerous empirical investigations have now linked deficits in ToM to a variety of behavioural, socio-emotional, and psychiatric difficulties in both children and adults (Pilowsky et al., 2000; Wang et al., 2008). Like many human competencies, ToM is thought to be the product of both social and biologically-based influences (Hughes et al., 2005). Despite independent lines of research suggesting both genetic and social contributions to ToM, no study has examined the joint effects of these factors on its development. The purpose of this study was to examine the interactive effect of previously implicated genetic and socialization factors on children’s ToM in the pre-school period. We focused on the oxytocin receptor gene (OXTR) and parenting behaviour, which are two of the most widely studied genetic and environmental contributors to ToM, respectively.
The biological basis of ToM
ToM undergoes a protracted development over the early years, with intuitive forms of reasoning in infancy maturing into more reflective forms of understanding in the pre-school period (Thoermer et al., 2012). Although no comprehensive life-course studies have been conducted, these findings suggest that the basic building blocks of ToM are present early in life, and may set the foundation for later development. This is consistent with so-called ‘nativist’ accounts of ToM, in which a biologically determined mechanism is believed to underlie this relatively modular ability (Leslie et al., 2004). To date, the most extensively studied neuropeptide involved in human social cognition is oxytocin (OXT). Studies in adult populations suggest that intranasal infusion of OXT improves emotion recognition (Di Simplicio et al., 2009) and the ability to infer others’ mental states from facial cues (Domes et al., 2007). Importantly, the behavioural effects of OXT depend on the distribution and expression of its receptor (OXTR), which is coded by the OXTR gene. Studies in non-human primates show that OXTR is selectively distributed in various brain regions known to support social cognition (Freeman et al., 2014). Moreover, it has been suggested that both the anatomy and functional physiology of the human nervous system may be shaped by OXT signalling pathways (Carter, 2014), leading to the idea that variability in OXTR may contribute to individual differences in social behaviour and cognition by modulating the neural circuits involved in processing socio-affective information (Meyer-Lindenberg and Tost, 2012). For this reason, OXTR is a reasonable candidate for examining molecular genetic influences on ToM.
Over the last decade, multiple studies have emerged that link variability in OXTR to social-cognitive traits such as prosocial temperament (Tost et al., 2010), altruism (Israel et al., 2009) and empathic responding (Rodrigues et al., 2009). Two recent studies have demonstrated that OXTR may modulate ToM specifically in both children and adults (Lucht et al., 2013; Wu and Su, 2013). Although results from behavioural genetic studies generally agree that at least some of the variability in ToM is genetically mediated (Hughes and Cutting, 1999; Ronald et al., 2006), there also appears to be significant shared and non-shared environmental influences (Hughes et al., 2005). Further, these studies insinuate that environmental effects may become more germane in middle compared with early childhood as children’s social horizons are broadened through caregiver, school and peer relations. Thus, during the pre-school period we might expect a confluence of genetic and environmental factors to be operative in shaping ToM.
Importantly, the functionality of OXTR single nucleotide polymorphisms (SNPs) remains largely unknown. However, Mizumoto et al. (1997) demonstrated that the third intronic region of OXTR is associated with transcriptional repression of the gene. In their study, differential methylation of a CpG island within this region was associated with relative gene expression in peripheral blood cells and myometrial cells; and recent studies suggest that these effects may also extend to OXTR expression in human cortex (Gregory et al., 2009). These results suggest that expression of OXTR may be epigentically regulated by DNA methylation, findings that potentially underpin the role of auxiliary molecular or environmental factors in OXTR functioning. Despite these fascinating findings, the specific environmental modulators of OXTR activity remain unexplored.
Parenting and ToM
For over two decades the developmental literature has shown that responsive parenting is one of the most robust influences on children’s ToM. These findings are consistent with ‘constructivist’ views of ToM, in which children’s understanding of others’ minds is thought to depend on critical social inputs which foster their mentalization skills (Carpendale and Lewis, 2004). To date, a host of specific parenting behaviours have been linked to children’s emerging ToM, including sensitivity, mental-state discourse, reflective capacities and mind-mindedness (Ereky-Stevens 2008; Laranjo et al., 2010; Meins et al., 2002). The overarching idea is that children benefit from social interactions because it affords them the alternative perspectives and symbolic material that are required to internalize, represent, and reason about others’ mental states (Fernyhough, 2008). From this perspective, while parental dispositions that are contingent, attuned, and warm may be important for engaging children in social exchanges, the cognitive ‘material’ that facilitates ToM is necessarily mind- and perspective-oriented (de Rosnay and Hughes, 2006). Thus, in this study we examine ‘cognitive sensitivity’, operationalized as parents’ ability to identify and respond to the cognitive needs and abilities of a child. This construct comprises three related skills: (i) promoting mutuality/reciprocity; (ii) mind-reading (thinking about what the child knows and understands); and (iii) communicative clarity (providing verbal/non-verbal input at a level appropriate for the child, and promoting a shared understanding of the goals of the task; see Prime et al., 2015).
Given the associations reported earlier, it is conceivable that cognitively sensitive parenting behaviours represent important sources of environmental variability that interact with inter-individual genetic variation in OXTR which pre-disposes to differential levels of ToM. In this study we attempt to bridge the nativist and constructivist views of ToM together by examining the potential interactive effect of OXTR variability and maternal cognitive sensitivity on children’s ToM development in the pre-school period, as this is the time at which ToM is most commonly and reliably measured (Wellman et al., 2001).
Current study
This study used a socioeconomically diverse sample of 301 pre-school-aged children (4.5 years) to test our gene–environment interaction model of ToM. We selected six OXTR SNPs: rs1042778, rs53576, rs2254298, rs11131149, rs237897 and rs237899. The first of these is located in the 3′-untranslated region (3′-UTR; exon 4) of the gene, and been suggested to play a regulatory role in OXTR transcription and translation (Israel et al., 2009). The other five SNPs tagged the large and potentially functional third intron of OXTR which, as mentioned earlier, contains a specific motif of 10–15 nucleotides that bind nuclear suppression proteins associated with down-regulation of the gene (Mizumoto et al., 1997). These SNPs were also chosen on the basis of their association with other social, behavioural and psychiatric phenotypes, which are now well characterized in the extant literature. Owing to their potential effect on transcriptional suppression, it was hypothesized that one or more of these SNPs would interact with maternal cognitive sensitivity to significantly predict children’s ToM at age 4.5.
Materials and Methods
Participants
This study was part of an ongoing longitudinal study (Kids, Families, Places study; iKFP) that aimed to investigate genetic and environmental influences on children’s social, cognitive and emotional development. All women giving birth in Toronto and Hamilton, ON, between April 2006 and September 2007 were considered for participation. Families were recruited through a program called ‘Healthy Babies Healthy Children’, which is a universal public health program to help with the transition to parenthood. Parents of all registered newborns were contacted within several days of the child’s birth. Inclusion criteria for the iKFP participation (which involved longitudinal follow-up and intensive observational measurement) included an English-speaking mother, a newborn >1500 g, at least two children <4 years old, and agreement to be filmed in the home. The University of Toronto Research Ethics Board approved all procedures, including informed consent.
At Time 1 (T1; Mage = 2.0 months; SD = 1.06), 501 families were enlisted in the study. Due to attrition, 397 (79.2%) families were followed up at Time 2 (T2; Mage = 1.60 years; SD = 0.16), 385 (76.8%) were followed up at Time 3 (T3; Mage = 3.15 years; SD = 0.27) and 323 (64.5%) were followed up at Time 4 (T4; Mage = 4.79 years; SD = 0.28). Attrition analysis showed that family dropout was related to lower maternal age at first pregnancy, t(494) = −5.10, P < 0.001, lower socioeconomic status (SES), t(498) =−5.07, P < 0.001, and lower maternal education, t(498) = −2.99, P < 0.005.
Of the total sample, 350 provided cheek swabs for DNA extraction. Outcome data for ToM were drawn from T4 (i.e. pre-school age; 4.5 years). In order to provide the most powerful test of genetic association (Laird and Lange, 2008), we also controlled for numerous covariates which were drawn from earlier time points. Of the 323 children participating at T4, no ToM information was available for 22 of them due to child non-compliance or tester administration error. This resulted in a final sample of 301 children.
We compared the study sample at T1 with the general population of Toronto and Hamilton using 2006 Canada Census Data. The study sample was similar to the general population in terms of family size (M = 4.52, SD = 1.01 compared with M = 4.13; SD = 1.22) and personal income ($30 000–39 999 vs census population mean = $30 504.16, SD = $37 808.12). Because the study sample was recruited shortly after childbirth, there were unsurprisingly fewer non-intact families (lone-parent: 5 vs 16.8%; step-families: 4.3 vs 10.3%). There were also fewer immigrants (47 vs 57.7%) and more educated mothers (53% had a Bachelor’s degree compared with 30.6% in the general population). Table 1 presents sample demographics at T4, which was the primary time point this study was based on.
Table 1.
Sample demographics of participants at T4
| Measure | n | % of sample |
|---|---|---|
| Ethnicity of mothers | 323 | 100.0 |
| European/Caucasian | 187 | 57.9 |
| South Asian | 45 | 13.9 |
| East Asian | 39 | 12.1 |
| Black | 21 | 6.5 |
| Other | 31 | 9.6 |
| Teen mom | 14 | 4.3 |
| Single parent family | 18 | 5.6 |
| New-immigrant family (<10 years) | 144 | 44.6 |
| Low income family (<$20 000) | 23 | 7.1 |
| Mother’s years of education (<high school) | 20 | 6.2 |
| Infant birth weight <2500 g | 9 | 2.8 |
Procedure
At each time point, a home visit of ∼2 h was conducted in which parents (usually the mother) filled out questionnaires pertaining to family life, sociodemographic information and child behaviour. From T2 to T4, children underwent a battery of observational and/or standardized tasks meant to assess multiple domains of functioning. One of these tasks measured their ToM, described below.
Measures
Theory of mind
This was assessed at T4 using the scale described by Wellman and Liu (2004), representing the most comprehensively validated test of ToM (Sabbagh and Seamans, 2008), including validation studies across cultures, languages, and in both typically and atypically developing children (Peterson et al., 2012). This scale presents a series of tasks that sequentially map onto children’s emerging ToM abilities, as determined by Guttmann-scaling methods. As children move through the scale, tasks become conceptually more difficult, and thus progression through the tasks reflects more advanced ToM understanding. The first three tasks assessed children’s understanding of diverse desires and beliefs, and knowledge and ignorance. This is followed by tasks that measure more sophisticated ToM reasoning such as belief-based emotion and real-apparent emotion. If children failed two consecutive tasks on the scale, testing was stopped. For all ToM tasks, stories were enacted for children with the use of puppets and props to aid in understanding. For each of the tasks, the child is given a score of 0 (fail) or 1 (pass). A total score across all tasks was computed, with higher scores representing higher ToM ability. A total score was preferred to a mean score because, based on the discontinue criteria, children who progress to higher levels of the scale could in fact have lower mean scores than children who evince less sophisticated abilities as a mean score depends on the number of items administered. A total score avoids this problem. Internal consistency was high (α = 0.87).
Maternal cognitive sensitivity
At T3 and T4, trained coders watched 5-min videotaped interactions of mothers and their children. Each dyad was instructed to use Duplo building blocks to build a picture of a design. The task requires cooperation because each person can only touch two colors of blocks even though four colors make up the design. A thin-slice methodology (Ambady et al., 2000) was used to code the interactions. Mothers were coded on 11 items, each on a 5-point Likert scale ranging from 1 (‘Not at all true’) to 5 (‘Very true’). Example items included: ‘Gives clear and specific verbal directions’; ‘Gives positive non-verbal directions’; ‘Gives positive feedback to reinforce his/her partner’; ‘Is sensitive to what partner knows and/or understands’; and ‘Is responsive to partner’s request for help, even those that are subtle and/or non-verbal’. A mean across the 11 items was taken and used as the final score at each time point. Internal consistency of the scale was good at T3 (α = 0.92) and T4 (α = 0.92). Inter-rater reliability was established by double coding a random selection of 20% of families. Inter-rater reliability was high at T3 (α = 0.84) and T4 (α = 0.86), and absolute agreement (single measures) was acceptable at both T3 (ICC = 0.88) and T4 (ICC = 0.78). In order to assess children’s general exposure to cognitively sensitive parenting during childhood, we averaged mothers’ scores across T3 and T4 (r = 0.44, P < 0.001). Further information on this measure, including construct validation, can be found in two papers by Prime et al., (2015).
Covariates
Based on previous studies demonstrating the association between certain demographic and contextual factors on ToM, a number of variables were covaried: (i) children’s concurrent age (T4) in years; (ii) children’s gender (0 = male; 1 = female); (iii) children’s birth weight (T1), reported in kilogram and grams; (iv) children’s receptive language ability (T4), measured by the Peabody Picture Vocabulary Test (Dunn and Dunn, 1997); (v) the number of children in the home < 18-years old; (vi) maternal education, assessed as the total number of years of formal schooling, not including kindergarten; and (vii) family SES, which was measured as a composite that included: (i) annual family income, assessed on a scale from 1 (‘no income’) to 16 (‘$105 000 or more’); (ii) number of rooms in the family’s residence; and (iii) whether the family owns/co-owns their house/apartment and/or a vehicle, even if still making payments (yes/no variables). These four variables were rescaled, standardized, and averaged to create a composite score for income/assets. Higher values represented higher SES.
Genotyping
DNA was extracted from cheek swab samples. We selected six SNPs in the OXTR gene by searching for confirmed polymorphisms in public databases (ABI database and the NCBI SNP database (http://www.ncbi.nlm.nih.gov/SNP) and for pre-designed assays from Applied Biosystems (ABI, Foster City, CA, USA, Assay-on-Demand by Applied Biosystems). These SNPs included rs1042778, rs53576, rs2254298, rs11131149, rs237897 and rs237899 which, according to Hapmap, tagged the major haplotypes of OXTR. These markers were genotyped with the ABI 7900-HT Sequence Detection System (SDS) (Applied Biosystems) using the TaqMan 5′ nuclease assay for allelic discrimination. One of these markers, rs53576, failed to genotype in >50% of the sample in the first round of genotyping. An attempt to retype this marker was made, but it remained problematic, and was thus dropped from the current analysis. However, as noted in Hapmap, rs53576 is in the same haplotype block as marker rs237897, which we successfully genotyped and used in the present analysis. Each of the remaining five SNPs and their genetic information for the sample is presented in Table 2. As seen in this table, another SNP, rs1042778, did not conform to Hardy-Weinberg equilibrium, and was thus not analyzed further. The PCR reactions (10-µl volume) contained 30 ng of genomic DNA, 10 µmol of TaqMan Universal PCR Master Mix (Applied Biosystems) and 0.25 µl of allelic discrimination mix (Applied Biosystems) containing 36 µM of each primer and 8 µM of each probe. The thermal cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 94°C for 15 s and the annealing temperature of 60°C for 1 min. Each 96 well plate contained two negative controls. Plates were then read on the ABI 7900HT SDS using the allelic discrimination end-point analysis mode of SDS software package version 2.0 (Applied Biosystems).
Table 2.
Characteristics of each SNP in the study sample
| SNP | Chr location | Gene position | Major allele | Minor allele | MAF | No. informative families | HWE P-value |
|---|---|---|---|---|---|---|---|
| rs1042778a | 8 794 544 | 3′-UTR | T | G | 0.400 | 88 | .036 |
| rs2254298 | 8 802 227 | Intron 3 | A | G | 0.122 | 61 | .120 |
| rs11131149 | 8 802 851 | Intron 3 | A | G | 0.316 | 87 | .071 |
| rs237897 | 8 808 284 | Intron 3 | A | G | 0.424 | 117 | .125 |
| rs237899 | 8 808 514 | Intron 3 | A | G | 0.329 | 105 | .064 |
SNP, single nucleotide polymorphism; Chr, chromosome; UTR, untranslated region; MAF, minor allele frequency; HWE, Hardy-Weinberg equilibrium.
Note. Informative families are those that make a non-zero contribution to the FBAT statistic. These families are included because of the usefulness of their genotypic information for the current markers. This is typically the number of families with at least one heterozygous parent.
aThis marker was dropped from subsequent analysis as it was not in HWE.
Statistical analysis
The analysis was carried out using the Pedigree-Based Association Test (PBAT) with the Golden Helix SVS program. The PBAT approach is robust to population admixture and stratification, meaning that population subgroups and differing allelic distributions across ethnicities do not pose the same problem they do for case-control designs. This is because, in the PBAT analysis, the alleles transmitted to probands are compared with the distribution of alleles expected among the offspring, conditional on the parental genotypes and based on the principles of Mendelian transmission (i.e. parents used as pseudo-controls). The result is a robust and unbiased test statistic that, when significant, allows inferences for both association and linkage. In this study, we tested an additive model in which genotypes were coded to reflect the number of copies of each allele for a given marker. The quantitative trait, ToM, was offset by the phenotypic mean for each person, thereby generating a phenotypic residual. This has been shown to improve the statistical power of the PBAT statistic (Lange et al., 2002). To further improve power, Laird and Lange (2008) suggest controlling for non-genomic factors known to be associated with the quantitative trait. Therefore, the PBAT statistic was also adjusted to account for the covariates listed earlier. Computation of the main genetic and interaction effect followed the procedure outlined by Vansteelandt et al. (2008). Briefly, the approach uses a semi-parametric model that has a non-parametric component for the main environmental effect, and is thus robust to the misspecification of the main environmental effect (which is not estimated; also see Moerkerke et al., 2010), and a parametric component for the main genetic effect and the gene–environement interaction effect, which are both estimated from the model. That is, we fit the model model E(Yij | Xij, Zij, Si) = µ(Zij, Si) + βSNPXij + βinterXijZij, where i indexes the family, j the offspring, Y is the trait (i.e. ToM), X is the additive coding of the genotype (0, 1 or 2 copies), Z is the environment (i.e. maternal cognitive sensitivity), S encodes the dependency on the parental genotypes, and µ(.) is a non-parametric function that encodes the dependence of the trait on the environmental exposure and parental genotype. A positive value for the interaction reflects an increase in ToM with more copies of that allele given exposure to the environmental variable (cognitive sensitivity); a negative value reflects the reverse relationship. For this study, alpha was set at P < 0.0125 (Bonferroni correction for the four usable SNPs).
Results
Descriptive statistics
Table 3 reports the bivariate correlations among study variables, including means and standard deviations. For the outcome phenotype, ToM, significant associations with age, gender, language, maternal education, family SES and the moderator variable, maternal cognitive sensitivity, emerged as significant.
Table 3.
Means, SDs and bivariate correlations among study variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | Mean | SD | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. ToM (T4) | 3.71 | 1.58 | ||||||||
| 2. Child age (T4) | 0.23*** | 4.79 | 0.28 | |||||||
| 3. Female gender | 0.14* | −0.05 | ||||||||
| 4. Birth weight (T1) | 0.09 | 0.00 | −0.12** | 3.41 | 0.51 | |||||
| 5. Child language (T4) | 0.38*** | 0.09 | −0.01 | 0.25*** | 104.0 | 14.2 | ||||
| 6. Number of kids in home (T4) | −0.05 | −0.03 | 0.00 | 0.05 | −.12* | 2.56 | 0.89 | |||
| 7. Maternal education (T1) | 0.12* | 0.01 | −0.04 | 0.10* | 0.25*** | −0.16** | 15.3 | 2.68 | ||
| 8. Family SES (T2–T4) | 0.14* | 0.08 | 0.02 | 0.13** | 0.34*** | −0.16** | 0.49*** | 0.00a | 0.78 | |
| 9. Maternal cognitive sensitivity (T3–T4) | 0.17** | 0.12* | 0.03 | 0.16** | 0.37*** | −0.12* | 0.30** | 0.45*** | 3.60 | 0.66 |
***P < 0.001, **P < 0.01, *P < 0.050
SES, socioeconomic status.
aThis value is a standardized score, hence the mean of zero.
Preliminary analysis
Computation of the interaction effect using PBAT assumes genotype-environment independence given the parents, which is a reasonable assumption in family-based gene–environment interaction tests (Umbach and Weinberg, 2000). To get some confirmation that this assumption was accurate, we performed a preliminary association analysis between the selected SNPs of OXTR and the environmental covariate, maternal cognitive sensitivity. This analysis showed that none of the SNPs were significantly associated with maternal cognitive sensitivity (all PBAT P’s > 0.10), supporting model assumptions.
Single marker analysis
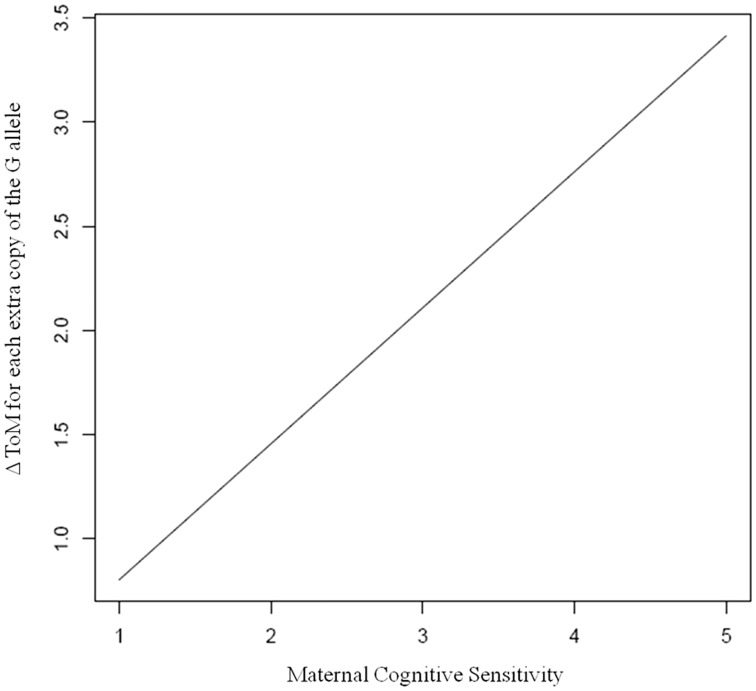
Table 4 shows the primary single marker association analyses, including main genetic effects and interactions of SNPs with maternal cognitive sensitivity. Results showed that, after controlling for covariates and the estimated interaction effect, there were no strong main genetic effects. The main effect between the rs11131149 marker and ToM was suggestive (PBAT P = 0.079), but did not survive correction for multiple comparisons. No other marker was significantly associated with ToM. After controlling for all covariates and the main genetic effects, there was a nominally significant interaction between the rs11131149 marker and maternal cognitive sensitivity [βinter = 0.65, 95% CI (0.12–1.18], z = 2.26, P = 0.019 for the major allele; βinter = −0.99, 95% CI (−1.81 to −.17), z = −2.39, P = 0.017 for the minor allele]. Because the coefficient for the major allele is positive, this means that, as the number of copies of G increases (from 0 to 1 to 2), ToM increases by an estimated 0.65 with more exposure to cognitively sensitive behaviour. This association narrowly missed the correction criteria for multiple comparisons. The heritability coefficient for this interaction was hinter = 0.26. The heritability coefficient reflects the proportion of variance in the outcome accounted for by the interaction, meaning that a substantial 26% of the variability in ToM was accounted for by the interaction between rs11131149 and cognitive sensitivity. There were no other significant interaction effects for the other OXTR markers1 (Figure 1).
Table 4.
Results of the gene and gene–environment interaction analysis for the SNPs with maternal cognitive sensitivity on theory of mind
| FBAT | QBAT |
QBAT-E |
|||||
|---|---|---|---|---|---|---|---|
| SNP | P-value | β [95% CI] | Z | P-value | β [95% CI] | Z | P-value |
| rs2254298 | |||||||
| G | 0.836 | −0.14 [−0.16, 0.35] | −0.54 | 0.582 | 0.54 [−1.26, 2.34] | 0.59 | 0.557 |
| A | −0.07 [−0.64, 0.50] | −0.24 | 0.807 | 0.06 [−0.35, 0.47] | 0.29 | 0.770 | |
| rs11131149 | |||||||
| G | 0.079* | 0.15 [−0.32, 0.62] | 0.63 | 0.534 | 0.65** [0.12, 1.18] | 2.36** | 0.019** |
| A | 0.10 [−0.43, 0.63] | 0.37 | 0.716 | −0.99** [−1.81, −0.17] | −2.39** | 0.017** | |
| rs237897 | |||||||
| G | 0.243 | 0.27 [−0.08, 0.62] | 1.50 | 0.133 | −0.08 [−0.53, 0.37] | −0.35 | 0.719 |
| A | −0.62 [−1.87, 0.63] | −0.41 | 0.134 | 0.25 [−1.81, 2.31] | 0.24 | 0.814 | |
| rs237899 | |||||||
| G | 0.301 | −0.51 [−1.24, 0.22] | −1.39 | 0.164 | −0.25 [−4.15, 3.65] | −0.13 | 0.899 |
| A | 0.35 [−0.18, 0.88] | 1.27 | 0.204 | 0.04 [−0.35, 0.43] | 0.20 | 0.856 | |
*P < 0.10. **P < 0.05.
SNP, single nucleotide polymorphism; FBAT, P-value for the FBAT statistic; FBAT, test for the main genetic effect in the model modeling only the main genetic effect (not the interaction). This is the primary test statistic for examining genetic associations in family-based designs. It is a generalization of the Transmission Disequilibrium Test method, in which alleles transmitted to affected offspring are compared with the expected distribution of alleles among offspring based on Mendel’s law of segregation.
QBAT, test for the main genetic effect in the gene–environment interaction model.
QBAT-E, test for the gene–environment interaction in the gene–environment interaction model.
β (95% CI), coefficient in the model for the main effect (QBAT) and gene–environment interaction (QBAT-E), and the 95% confidence interval; Z – [β/SE(β)], the value of the test statistic.
Fig. 1.
Change in ToM for each extra copy of the rs11131149 G allele (y-axis) as a function of maternal cognitive sensitivity (x-axis). The y-axis reflects the function , where is the main effect of the SNP and is the gene–environment interaction effect. In this plot, there is an increasing slope upward. This means that if you have the G allele of the rs11131149 SNP, with more exposure to the environmental variable, you have an increasing amount of ToM from 0 copies to 1 copy to 2 copies of the allele. If there were no interaction, the line would be perfectly horizontal. In a traditional interaction model, the relationship between the environmental covariate and the outcome phenotype would be plotted separately at different levels of the genotype, producing two separate intersecting lines (which were not possible here because of the semi-parametric model and the fact that the environmental effect is not estimated). The interaction plotted here is akin to taking the difference between these two lines. If there was no G*E, then the lines would be a vertical translation of each other. This is the typical approach for plotting an interaction using family-based methodology in an additive genetic model, as outlined by Vansteelandt et al. (2008).
Haplotype analysis
We conducted haplotype analysis using PBAT’s haplotype function. Linkage disequilibrium was established between rs11131149 and rs2254298, as well as between rs237897 and rs237899 (D′ > 0.8 for both). Haplotype tests of association between these two-marker haplotypes were generally less suggestive than the single-marker analysis. Specifically, for the rs11131149—rs2254298 haplotype (G/A allelic combination), there was a nominally significant interaction with maternal cognitive sensitivity on ToM (P = 0.027). Because the interaction effect of the haplotype was weaker than the interaction effect of rs11131149 alone, we suspect the haplotype does not explain anything more than the rs1131149 SNP. Also, there was no significant main genetic effect of this haplotype (FBAT P = 0.14). For the rs237897—rs237899 haplotype (A/A allelic combination), there was a nominally significant association with ToM (FBAT P = 0.037); however, the interaction between this haplotype and cognitive sensitivity was not shown to significantly predict ToM (z = 0.01, P = 0.99).
Discussion
This study aimed to examine a gene–environment interaction model of ToM in pre-school-aged children. The OXTR gene and maternal cognitive sensitivity were selected as the genetic and environmental factors owing to their known relationship to social cognition. It was suggestive that, despite no strong main genetic effects, one particular OXTR variant, rs11131149, interacted with maternal cognitive sensitivity to predict ToM ability in children at age 4.5. We also demonstrated a relatively weaker interaction between a haplotype consisting of rs11131149—rs2254298 (G/A) and cognitive sensitivity on ToM. Both of these markers (rs11131149 and rs2254298) are located within intron 3 of OXTR, a region that may regulate gene expression through epigenetic control mechanisms (Mizumoto et al. 1997). The current findings provide further empirical support for the notion that the operation of the genome is an emergent property that may be contingent upon environmental regulation, and extends recent calls for additional gene–environment interaction research in the field of child development (Meaney, 2010). These results are also consistent with studies in non-human species which show that effects of maternal care can become physically imprinted and directly alter intracellular activity that affects gene expression in offspring (Meaney, 2010).
The mechanisms through which OXTR influence human behaviour are complex, though there is mounting evidence that brain structure and function serve as an endophenotypic link between genes and behaviour (Yamasue, 2013). Imaging genetic studies demonstrate that there are several neural correlates of OXTR polymorphisms, including the amygdala, dorsal anterior cingulate gyrus and hypothalamus (Inoue et al., 2010; Tost et al., 2010), regions that are widely implicated in social understanding and cognition. Bio-evolutionary accounts of human brain development posit that OXT has played a central role in the encephalization of neocortex that supports cognitive development (Carter, 2014). For instance, OXT may directly facilitate cortical development by encouraging stem cell differentiation or inhibiting programmed death of brain cells (Gutkowska and Jankowski, 2012; Leuner et al., 2012). Alternatively, OXT may indirectly promote neurogenesis by tuning the nervous system to socialization factors that foster brain development (Adolphs, 2009). This includes, but is not limited to, prolonged infant care that promotes social learning and the establishment of interpersonal bonds that underlie social-cognitive development (Shultz and Dunbar 2010). Thus, variability in, and expression of, OXTR SNPs may have downstream effects on structural and functional neuroanatomy through the genetic modulation of OXT activity (Ebstein et al. 2012; Meyer-Lindenberg and Tost, 2012; Bethlehem et al., 2013).
Few previous studies have implicated the rs11131149 marker in human cognition or behaviour. However, a recent study showed that this marker is significantly associated with social cognition in children at 18 months (Wade et al., 2014), which is consistent with the purported continuity and maturation in social cognition from early to middle childhood. Moreover, the rs11131149 marker has been previously linked to the risk for autism spectrum disorder (Liu et al., 2010) and depressive temperament (Kawamura et al., 2010), conditions characterized by deficits in ToM. In the field of child psychiatry, gene–environment interaction effects have proven to be variable and complex (Caspi and Moffitt, 2006), with inconsistencies across studies often attributable to sample and/or methodological differences. Another explanation for cross-study inconsistencies is that there is heterogeneity in the phenomenological clustering of symptoms that define psychopathological conditions. That is, particular neurocognitive or psychological deficits may be present in some individuals but not others, who nonetheless meet diagnostic criteria based on pre-defined symptom counts and cut-off scores. For this reason, uncovering the specific cognitive endophenotypes that are associated with particular genotypes and genotype-environment interactions may help to further specify the cognitive and behavioural traits that are impaired in complex psychiatric conditions (Hyde et al., 2011). Notwithstanding the need for replication, the current results are noteworthy because they suggest that genotype-environment interactions may operate for ToM, a discrete cognitive endophenotype that is associated with multiple psychopathological conditions. In this study it was suggestive that as the number of copies of the major allele of the rs11131149 marker increased, ToM also increased as a function of exposure to higher levels of maternal cognitive sensitivity. However, we did not demonstrate a strong main genetic effect of this or any other marker. This lack of main genetic effect suggests that, indeed, the effect of particular genetic variants on children’s ToM development may crucially depend on auxiliary environmental factors. The lack of direct genetic effect on behavioural and psychological traits may be obscured if such effects are only operational in pre-disposing environments (Manuck and McCaffery, 2014). In fact, most individual SNPs account for an extremely small proportion of the phenotypic variance, usually on the order of 1% (Plomin, 2013); whereas twin-studies suggest that upwards of 50% of individual differences are genetically mediated (Turkheimer, 2000). This problem of ‘missing heritability’ is manifold, but at least some is believed to be contained in conditionally expressed genetic variation (Plomin, 2013). In this study, a considerable 26% of the variability in ToM was accounted for the interaction of rs11131149 with maternal cognitive sensitivity, a finding consistent with the idea that gene associations may be amplified when examined in the context of particular environmental conditions (Manuck and McCaffery, 2014).
Gene–environment interactions are suggestive that environmental conditions moderate gene expression or, alternatively, genetic factors pre-dispose to the negative (or positive) effects of environmental adversity (or enrichment). Although not examined in this study, it has been demonstrated that OXTR can be epigenetically silenced through DNA methylation (Mizumoto et al., 1997; Gregory et al., 2009). Gregory et al. (2009) showed reduced OXTR mRNA expression in temporal cortical areas in association with increased methylation in individuals with autism, a condition characterized by pronounced deficits in ToM. Building on the putative role of OXTR in the organization of the nervous system, Jack et al. (2012) showed that OXTR hypermethylation was associated with activity in two regions—one extending from superior temporal gyrus to supramarginal gyrus at the temporo-parietal junction, and a second in the dorsal anterior cingulate cortex. These are two of the most widely cited areas involved in mental state attribution and ToM (Gallagher and Frith, 2003; Völlm et al., 2006; Spreng et al., 2009). Indeed, these areas may form a network with other limbic (e.g. amygdala) and pre-frontal regions for representation of self and other that underlies ToM (Abu-Akel, 2003; Mar, 2011). Although future research is needed, it may be the case that epigentically regulated OXTR expression plays a role in ToM through its effect on neural activity in the brain regions that support social engagement and understanding (e.g. Johnson et al., 2009; Kumsta et al., 2013; Yamasue, 2013).
Finally, while this study demonstrated that OXTR interacts with parenting behaviour on children’s ToM, separate lines of research have shown that mothers’ OXTR genotype is associated with her own parenting behaviour (Bakermans-Kranenburg et al., 2008; Feldman et al., 2012; Michalska et al., 2014). Thus, it is possible that mothers with particular genetic dispositions exhibit more optimal forms of caregiving, which in combination with the child’s own genotype, is related to improved social cognition. Further, a recent study demonstrated an evocative gene–environment correlation between child OXTR genotype and a measure of parenting confidence, suggesting that children’s genetics may recruit certain elements of parenting through genetically mediated child behaviours (Kryski et al., 2014). Importantly, our measure of cognitive sensitivity is quite different from the metric of parenting confidence in the latter study, and indeed we did not demonstrate a gene–environment correlation between child OXTR and maternal cognitive sensitivity. Nevertheless, together these findings suggest the possibility that both gene–environment interactions and gene–environment correlations may be operative in parent–child relations and/or children’s cognitive outcomes. Uncovering and mapping the specific pathways to social behaviour through genetic and environmental conditions is a ripe area for future research.
Limitations
The primary limitation of this study is a lack of replication sample, which lends to the possibility of spurious associations. Aside this drawback, this study abided by the recommended guidelines for candidate gene studies (Johnston et al., 2013). Future replication studies are encouraged to enhance our understanding of the genetic and gene–environment relationship between OXTR and social cognition in childhood and beyond.
Acknowledgements
We are grateful to the families who give so generously of their time, to the Hamilton and Toronto Public Health Units for facilitating recruitment of the sample, and to Mira Boskovic for project management. The study team, beyond the current authors includes: Cathy Barr, Kathy Georgiades, Greg Moran, Tom O’Connor, Michal Perlman, Hildy Ross, Louis Schmidt. Thanks to Cathy Barr for her comments on earlier versions of this article.
Footnotes
1 As there was an association between gender and ToM (female advantage), we examined gender differences in the genetic effects. First, there were no differences in the distribution of individual alleles across gender for any of the markers (all χ2 [df = 1] < 2.70, P’s > .10), nor were there any gender differences in genotype makeup (all χ2 [df = 2] < 3.5, P’s > 0.10). However, we did observe a modest gene*gender interaction on ToM for the rs11131149 marker only (zinter = 2.08, P = 0.04). This interaction was consistent with the suggestion that more copies of the major allele (G) were associated with higher ToM in females compared with males. However, no evidence for a three-way interaction between gene*gender*cognitive sensitivity emerged (zinter = 0.85, P = 0.39). Thus, although there is a modest gene*gender interaction on ToM, it does not appear that the documented interaction between OXTR and maternal cognitive sensitivity on ToM varies as a function of gender in this study.
Funding
The grant ‘Transactional Processes in Emotional and Behavioral Regulation: Individuals in Context’ was awarded to Jennifer M. Jenkins and Michael Boyle from the Canadian Institutes of Health Research (Funding Reference No. 70334) and covered data collection.
Conflict of interest. None declared.
References
- Abu-Akel A. (2003). A neurobiological mapping of theory of mind. Brain Research Reviews, 43(1), 29–40. [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology, 60, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambady N., Bernieri F.J., Richeson J.A. (2000). Toward a histology of social behavior: Judgmental accuracy from thin slices of the behavioral stream. Advances in Experimental Social Psychology , 32, 201–71. [Google Scholar]
- Bakermans-Kranenburg M.J., van IJzendoorn M.H. (2008). Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience, 3(2), 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Lombardo M., Tager-Flusberg H., Cohen D. (2013). Understanding Other Minds: Perspectives from developmental social neuroscience, Oxford: Oxford University Press. [Google Scholar]
- Bethlehem R.A., van Honk J., Auyeung B., Baron-Cohen S. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38(7), 962–74. [DOI] [PubMed] [Google Scholar]
- Carpendale J.I., Lewis C. (2004). Constructing an understanding of mind: The development of children’s social understanding within social interaction. Behavioral and Brain Sciences , 27(1), 79–96. [DOI] [PubMed] [Google Scholar]
- Carter C.S. (2014). Oxytocin pathways and the evolution of human behavior. Annual Review of Psychology, 65, 17–39. [DOI] [PubMed] [Google Scholar]
- Caspi A., Moffitt T.E. (2006). Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature Reviews Neuroscience , 7(7), 583–90. [DOI] [PubMed] [Google Scholar]
- de Rosnay M., Hughes C. (2006). Conversation and theory of mind: do children talk their way to socio-cognitive understanding? British Journal of Developmental Psychology , 24(1), 7–37. [Google Scholar]
- Di Simplicio M., Massey-Chase R., Cowen P., Harmer C. (2009). Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. Journal of Psychopharmacology , 23(3), 241–8. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. (2007). Oxytocin improves “mind-reading” in humans. Biological Psychiatry , 61(6), 731–3. [DOI] [PubMed] [Google Scholar]
- Dunn L.M., Dunn L.M. (1997). Peabody Picture Vocabulary Test-Third edition: Manual. Circle Pines, MN: American Guidance Services. [Google Scholar]
- Ebstein R.P., Knafo A., Mankuta D., Chew S.H., Lai P.S. (2012). The contributions of oxytocin and vasopressin pathway genes to human behavior. Hormones and Behavior, 61(3), 359–79. [DOI] [PubMed] [Google Scholar]
- Ereky-Stevens K. (2008). Associations between mothers’ sensitivity to their infants’ internal states and children's later understanding of mind and emotion. Infant and Child Development , 17(5), 527–43. [Google Scholar]
- Feldman R., Zagoory-Sharon O., Weisman O., et al. (2012). Sensitive Parenting Is Associated with Plasma Oxytocin and Polymorphisms in the OXTR and CD38 Genes. Biological Psychiatry, 72(3), 175–81. [DOI] [PubMed] [Google Scholar]
- Fernyhough C. (2008). Getting Vygotskian about theory of mind: mediation, dialogue, and the development of social understanding. Developmental Review , 28(2), 225–62. [Google Scholar]
- Freeman S.M., Inoue K., Smith A.L., Goodman M.M., Young L.J. (2014). The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology , 45, 128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L., Frith C.D. (2003). Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences, 7(2), 77–83. [DOI] [PubMed] [Google Scholar]
- Gregory S.G., Connelly J.J., Towers A.J., et al. (2009). Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Medicine , 7(1), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkowska J., Jankowski M. (2012). Oxytocin revisited: its role in cardiovascular regulation. Journal of Neuroendocrinology, 24(4), 599–608. [DOI] [PubMed] [Google Scholar]
- Hughes C., Cutting A.L. (1999). Nature, nurture, and individual differences in early understanding of mind. Psychological Science, 10(5), 429–32. [Google Scholar]
- Hughes C., Jaffee S.R., Happe F., Taylor A., Caspi A., Moffitt T.E. (2005). Origins of individual differences in theory of mind: From nature to nurture?. Child Development , 76(2), 356–70. [DOI] [PubMed] [Google Scholar]
- Hyde L.W., Bogdan R., Hariri A.R. (2011). Understanding risk for psychopathology through imaging gene-environment interactions. Trends in Cognitive Sciences , 15(9), 417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Yamasue H., Tochigi M., et al. (2010). Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biological Psychiatry, 68(11), 1066–72. [DOI] [PubMed] [Google Scholar]
- Israel S., Lerer E., Shalev I., et al. (2009). The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PloS One , 4(5), e5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A., Connelly J.J., Morris J.P. (2012). DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Frontiers in Human Neuroscience, 6, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Grossmann T., Kadosh K.C. (2009). Mapping functional brain development: Building a social brain through interactive specialization. Developmental Psychology, 45(1), 151. [DOI] [PubMed] [Google Scholar]
- Johnston C., Lahey B.B., Matthys W. (2013). Editorial policy for candidate gene studies. Journal of Abnormal Child Psychology, 41, 511–4. [Google Scholar]
- Kawamura Y., Liu X., Akiyama T., et al. (2010). The association between oxytocin receptor gene (OXTR) polymorphisms and affective temperaments, as measured by TEMPS-A. Journal of Affective Disorders , 127(1), 31-37. [DOI] [PubMed] [Google Scholar]
- Kryski K.R., Smith H.J., Sheikh H.I., Singh S.M., Hayden E.P. (2014). Evidence for evocative gene–environment correlation between child oxytocin receptor (OXTR) genotype and caregiver behavior. Personality and Individual Differences, 64, 107–10. [Google Scholar]
- Kumsta R., Hummel E., Chen F.S., Heinrichs M. (2013). Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Frontiers in Neuroscience, 7, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird N.M., Lange C. (2008). Family-based methods for linkage and association analysis. Advances in Genetics , 60, 219–52. [DOI] [PubMed] [Google Scholar]
- Lange C., DeMeo D.L., Laird N.M. (2002). Power and design considerations for a general class of family-based association tests: quantitative traits. The American Journal of Human Genetics , 71(6), 1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjo J., Bernier A., Meins E., Carlson S.M. (2010). Early manifestations of children’s theory of mind: the roles of maternal mind-mindedness and infant security of attachment. Infancy , 15(3), 300–23. [DOI] [PubMed] [Google Scholar]
- Leslie A.M., Friedman O., German T.P. (2004). Core mechanisms in ‘theory of mind’. Trends in Cognitive Sciences , 8(12), 528–33. [DOI] [PubMed] [Google Scholar]
- Leuner B., Caponiti J.M., Gould E. (2012). Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus, 22(4), 861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Kawamura Y., Shimada T., et al. (2010). Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. Journal of Human Genetics , 55(3), 137–41. [DOI] [PubMed] [Google Scholar]
- Lucht M.J., Barnow S., Sonnenfeld C., et al. (2013). Associations between the oxytocin receptor gene (OXTR) and “mind-reading” in humans-an exploratory study. Nordic Journal of Psychiatry , 67(1), 15–21. [DOI] [PubMed] [Google Scholar]
- Manuck S.B., McCaffery J.M. (2014). Gene-environment interaction. Annual Review of Psychology , 65, 41–70. [DOI] [PubMed] [Google Scholar]
- Mar R.A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–34. [DOI] [PubMed] [Google Scholar]
- Meaney M.J. (2010). Epigenetics and the biological definition of gene—environment interactions. Child Development , 81(1), 41–79. [DOI] [PubMed] [Google Scholar]
- Meins E., Fernyhough C., Wainwright R., Das Gupta M., Fradley E., Tuckey M. (2002). Maternal mind-mindedness and attachment security as predictors of theory of mind understanding. Child Development , 73(6), 1715–26. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Tost H. (2012). Neural mechanisms of social risk for psychiatric disorders. Nature Neuroscience, 15(5), 663–8. [DOI] [PubMed] [Google Scholar]
- Michalska K.J., Decety J., Liu C., et al. (2014). Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Frontiers in Behavioral Neuroscience, 8, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto Y., Kimura T., Ivell R. (1997). A genomic element within the third intron of the human oxytocin receptor gene may be involved in transcriptional suppression. Molecular and Cellular Endocrinology , 135(2), 129–38. [DOI] [PubMed] [Google Scholar]
- Moerkerke B., Vansteelandt S., Lange C. (2010). A doubly robust test for gene–environment interaction in family-based studies of affected offspring. Biostatistics, 11(2), 213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C.C., Wellman H.M., Slaughter V. (2012). The mind behind the message: advancing theory-of-mind scales for typically developing children, and those with deafness, autism, or asperger syndrome. Child Development, 83(2), 469–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky T., Yirmiya N., Arbelle S., Mozes T. (2000). Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophrenia Research , 42(2), 145–55. [DOI] [PubMed] [Google Scholar]
- Plomin R. (2013). Child development and molecular genetics: 14 years later. Child Development , 84(1), 104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime H., Browne D., Akbari E., Wade M., Madigan S., Jenkins J.M. (2015). The development of a measure of caregiver cognitive sensitivity appropriate for use in primary care health settings. Journal of Child Psychology and Psychiatry, 56(4), 488–95. [DOI] [PubMed] [Google Scholar]
- Prime H., Perlman M., Tackett J., Jenkins J.M. (2013). Cognitive sensitivity in sibling interactions: development of the construct and comparison of two coding methodologies. Early Education and Development, 25(2), 240–58. [Google Scholar]
- Rodrigues S.M., Saslow L.R., Garcia N., John O.P., Keltner D. (2009). Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences , 106(50), 21437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A., Viding E., Happé F., Plomin R. (2006). Individual differences in theory of mind ability in middle childhood and links with verbal ability and autistic traits: a twin study. Social Neuroscience, 1(3–4), 412–25. [DOI] [PubMed] [Google Scholar]
- Sabbagh M.A., Seamans E.L. (2008). Intergenerational transmission of theory-of-mind. Developmental Science, 11(3), 354–60. [DOI] [PubMed] [Google Scholar]
- Shultz S., Dunbar R. (2010). Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proceedings of the National Academy of Sciences, 107(50), 21582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Thoermer C., Sodian B., Vuori M., Perst H., Kristen S. (2012). Continuity from an implicit to an explicit understanding of false belief from infancy to preschool age. British Journal of Developmental Psychology , 30(1), 172–87. [DOI] [PubMed] [Google Scholar]
- Tost H., Kolachana B., Hakimi S., et al. (2010). A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences , 107(31), 13936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E. (2000). Three laws of behavior genetics and what they mean. Current Directions in Psychological Science , 9(5), 160–4. [Google Scholar]
- Umbach D.M., Weinberg C.R. (2000). The use of case-parent triads to study joint effects of genotype and exposure. The American Journal of Human Genetics , 66(1), 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteelandt S., DeMeo D.L., Lasky-Su J., et al. (2008). Testing and estimating gene-environment interactions in family-based association studies. Biometrics , 64(2), 458–67. [DOI] [PubMed] [Google Scholar]
- Völlm B.A., Taylor A.N., Richardson P., et al. (2006). Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage, 29(1), 90–8. [DOI] [PubMed] [Google Scholar]
- Wade M., Hoffmann T., Wigg K., Jenkins J.M. (2014). Association between the oxytocin receptor gene and children’s social cognition at 18 months. Genes, Brain and Behavior , 13(7), 603–10. [DOI] [PubMed] [Google Scholar]
- Wang Y.-G., Wang Y.-Q., Chen S.-L., Zhu C.-Y., Wang K. (2008). Theory of mind disability in major depression with or without psychotic symptoms: a componential view. Psychiatry Research , 161(2), 153–61. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Cross D., Watson J. (2001). Meta-analysis of theory-of-mind development: the truth about false belief. Child Development, 72(3), 655–84. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Liu D. (2004). Scaling of theory-of-mind tasks. Child Development, 75(2), 523–41. [DOI] [PubMed] [Google Scholar]
- Wu N., Su Y. (2013). Oxytocin receptor gene relates to theory of mind and prosocial behavior in children. Journal of Cognition and Development, 16(2), 302–13. [Google Scholar]
- Yamasue H. (2013). Function and structure in social brain regions can link oxytocin-receptor genes with autistic social behavior. Brain and Development, 35(2), 111–8. [DOI] [PubMed] [Google Scholar]