Abstract
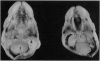
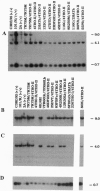
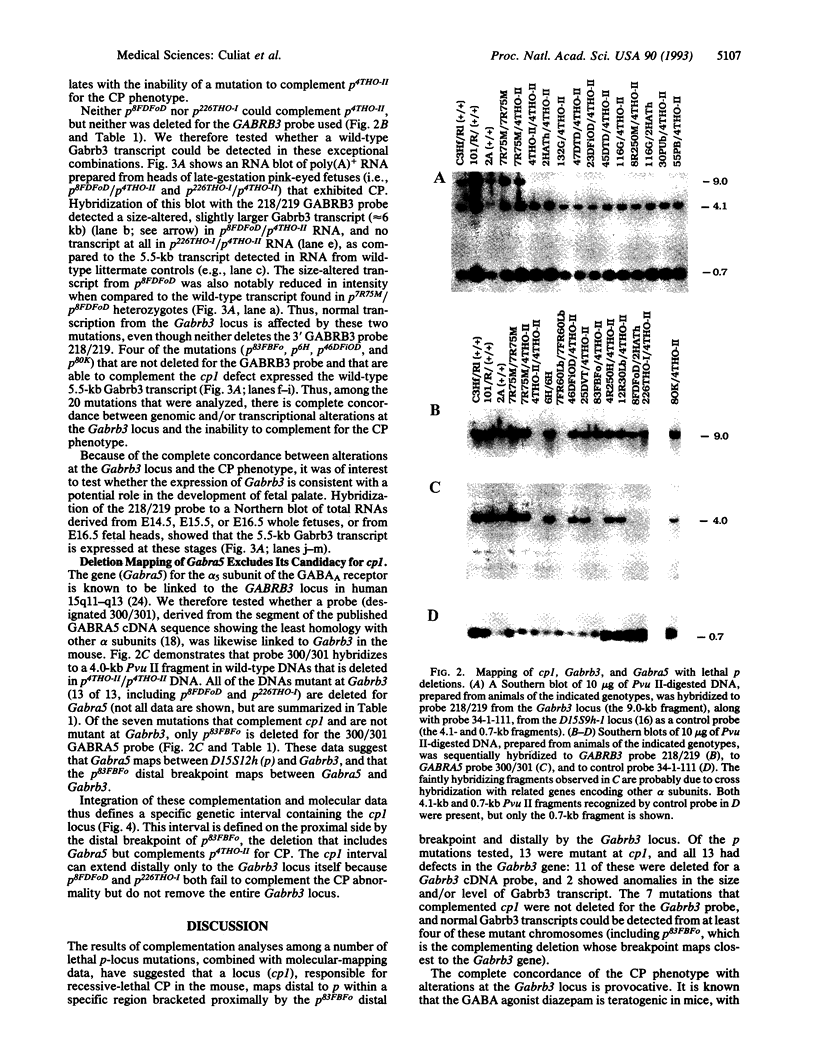
Genetic and molecular analyses of a number of radiation-induced deletion mutations of the pink-eyed dilution (p) locus in mouse chromosome 7 have identified a specific interval on the genetic map associated with a neonatally lethal mutation that results in cleft palate. This interval, closely linked and distal to p, and bracketed by the genes encoding the alpha 5 and beta 3 subunits of the type A gamma-aminobutyric acid receptor (Gabra5 and Gabrb3, respectively), contains a gene(s) (cp1; cleft palate 1) necessary for normal palate development. The cp1 interval extends from the distal breakpoint of the prenatally lethal p83FBFo deletion to the Gabrb3 locus. Among 20 p deletions tested, there was complete concordance between alterations at the Gabrb3 transcription unit and inability to complement the cleft-palate defect. These mapping data, along with previously described in vivo and in vitro teratological effects of gamma-aminobutyric acid or its agonists on palate development, suggest the possibility that a particular type A gamma-aminobutyric acid receptor that includes the beta 3 subunit may be necessary for normal palate development. The placement of the cp1 gene within a defined segment of the larger D15S12h (p)-D15S9h-1 interval in the mouse suggests that the highly homologous region of the human genome, 15q11-q13, be evaluated for a role(s) in human fetal facial development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardinger H. H., Buetow K. H., Bell G. I., Bardach J., VanDemark D. R., Murray J. C. Association of genetic variation of the transforming growth factor-alpha gene with cleft lip and palate. Am J Hum Genet. 1989 Sep;45(3):348–353. [PMC free article] [PubMed] [Google Scholar]
- Brilliant M. H. The mouse pink-eyed dilution locus: a model for aspects of Prader-Willi syndrome, Angelman syndrome, and a form of hypomelanosis of Ito. Mamm Genome. 1992;3(4):187–191. doi: 10.1007/BF00355717. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Barr J. A., Evans E. P., Burtenshaw M., Beechey C. V., Leff S. E., Brannan C. I., Copeland N. G., Jenkins N. A., Jones J. A candidate mouse model for Prader-Willi syndrome which shows an absence of Snrpn expression. Nat Genet. 1992 Dec;2(4):270–274. doi: 10.1038/ng1292-270. [DOI] [PubMed] [Google Scholar]
- Chenevix-Trench G., Jones K., Green A. C., Duffy D. L., Martin N. G. Cleft lip with or without cleft palate: associations with transforming growth factor alpha and retinoic acid receptor loci. Am J Hum Genet. 1992 Dec;51(6):1377–1385. [PMC free article] [PubMed] [Google Scholar]
- Chung C. S., Bixler D., Watanabe T., Koguchi H., Fogh-Andersen P. Segregation analysis of cleft lip with or without cleft palate: a comparison of Danish and Japanese data. Am J Hum Genet. 1986 Nov;39(5):603–611. [PMC free article] [PubMed] [Google Scholar]
- Fraser F. C. Mapping the cleft-lip genes: the first fix? Am J Hum Genet. 1989 Sep;45(3):345–347. [PMC free article] [PubMed] [Google Scholar]
- Fritschy J. M., Benke D., Mertens S., Oertel W. H., Bachi T., Möhler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski S. M., Adams K. J., Birch P. H., Friedman J. M., Goodfellow P. J. The gene responsible for X-linked cleft palate (CPX) in a British Columbia native kindred is localized between PGK1 and DXYS1. Am J Hum Genet. 1992 May;50(5):1129–1136. [PMC free article] [PubMed] [Google Scholar]
- Hecht J. T., Wang Y. P., Blanton S. H., Michels V. V., Daiger S. P. Cleft lip and palate: no evidence of linkage to transforming growth factor alpha. Am J Hum Genet. 1991 Sep;49(3):682–686. [PMC free article] [PubMed] [Google Scholar]
- Johnston M. C., Bronsky P. T. Animal models for human craniofacial malformations. J Craniofac Genet Dev Biol. 1991 Oct-Dec;11(4):277–291. [PubMed] [Google Scholar]
- Kalter H. The history of the A family of inbred mice and the biology of its congenital malformations. Teratology. 1979 Oct;20(2):213–232. doi: 10.1002/tera.1420200206. [DOI] [PubMed] [Google Scholar]
- Karolyi J., Erickson R. P., Liu S., Killewald L. Major effects on teratogen-induced facial clefting in mice determined by a single genetic region. Genetics. 1990 Sep;126(1):201–205. doi: 10.1093/genetics/126.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khrestchatisky M., MacLennan A. J., Chiang M. Y., Xu W. T., Jackson M. B., Brecha N., Sternini C., Olsen R. W., Tobin A. J. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989 Dec;3(6):745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Knoll J. H., Sinnett D., Wagstaff J., Glatt K., Wilcox A. S., Whiting P. M., Wingrove P., Sikela J. M., Lalande M. FISH ordering of reference markers and of the gene for the alpha 5 subunit of the gamma-aminobutyric acid receptor (GABRA5) within the Angelman and Prader-Willi syndrome chromosomal regions. Hum Mol Genet. 1993 Feb;2(2):183–189. doi: 10.1093/hmg/2.2.183. [DOI] [PubMed] [Google Scholar]
- Kuwano A., Mutirangura A., Dittrich B., Buiting K., Horsthemke B., Saitoh S., Niikawa N., Ledbetter S. A., Greenberg F., Chinault A. C. Molecular dissection of the Prader-Willi/Angelman syndrome region (15q11-13) by YAC cloning and FISH analysis. Hum Mol Genet. 1992 Sep;1(6):417–425. doi: 10.1093/hmg/1.6.417. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Wisden W., Seeburg P. H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992 Nov;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F., King T. R., Gondo Y., Gardner J. M., Nakatsu Y., Eicher E. M., Brilliant M. H. Genetic and molecular analysis of recessive alleles at the pink-eyed dilution (p) locus of the mouse. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6968–6972. doi: 10.1073/pnas.89.15.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. P., Becker B. A. Teratogenicity of oral diazepam and diphenylhydantoin in mice. Toxicol Appl Pharmacol. 1975 Apr;32(1):53–61. doi: 10.1016/0041-008x(75)90194-5. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D., Gottlieb W., Russell L. B., Davda M., Horsthemke B., Rinchik E. M. Evaluation of potential models for imprinted and nonimprinted components of human chromosome 15q11-q13 syndromes by fine-structure homology mapping in the mouse. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2050–2054. doi: 10.1073/pnas.90.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Reis A., Kunze J., Ladanyi L., Enders H., Klein-Vogler U., Niemann G. Exclusion of the GABAA-receptor beta 3 subunit gene as the Angelman's syndrome gene. Lancet. 1993 Jan 9;341(8837):122–123. doi: 10.1016/0140-6736(93)92606-t. [DOI] [PubMed] [Google Scholar]
- Saitoh S., Kubota T., Ohta T., Jinno Y., Niikawa N., Sugimoto T., Wagstaff J., Lalande M. Familial Angelman syndrome caused by imprinted submicroscopic deletion encompassing GABAA receptor beta 3-subunit gene. Lancet. 1992 Feb 8;339(8789):366–367. doi: 10.1016/0140-6736(92)91686-3. [DOI] [PubMed] [Google Scholar]
- Wagstaff J., Knoll J. H., Fleming J., Kirkness E. F., Martin-Gallardo A., Greenberg F., Graham J. M., Jr, Menninger J., Ward D., Venter J. C. Localization of the gene encoding the GABAA receptor beta 3 subunit to the Angelman/Prader-Willi region of human chromosome 15. Am J Hum Genet. 1991 Aug;49(2):330–337. [PMC free article] [PubMed] [Google Scholar]
- Wee E. L., Norman E. J., Zimmerman E. F. Presence of gamma-aminobutyric acid in embryonic palates of AJ and SWV mouse strains. J Craniofac Genet Dev Biol. 1986;6(1):53–61. [PubMed] [Google Scholar]
- Wee E. L., Zimmerman E. F. Involvement of GABA in palate morphogenesis and its relation to diazepam teratogenesis in two mouse strains. Teratology. 1983 Aug;28(1):15–22. doi: 10.1002/tera.1420280104. [DOI] [PubMed] [Google Scholar]