Fig. 2.
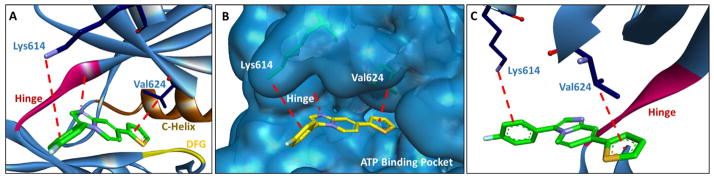
Computational modeling of 3 (17) in FLT3. (A) 3 (17) creates key interactions with Lys614 (cation-pi), Val624 (sigma-pi), and the hinge. (B) Space filling diagram depicting the FLT3 active site in complex with 3 (17). From the modeling study, the inhibitor appears to be directly competitive with ATP for the ATP binding pocket. (C) Alternative angle to (A) to help better illustrate interactions with Lys614, Val624, and the hinge. The hinge is shown in red, the c-helix in brown, and the DFG motif in yellow. PDB# 1RJB [32]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)