Figure 3.
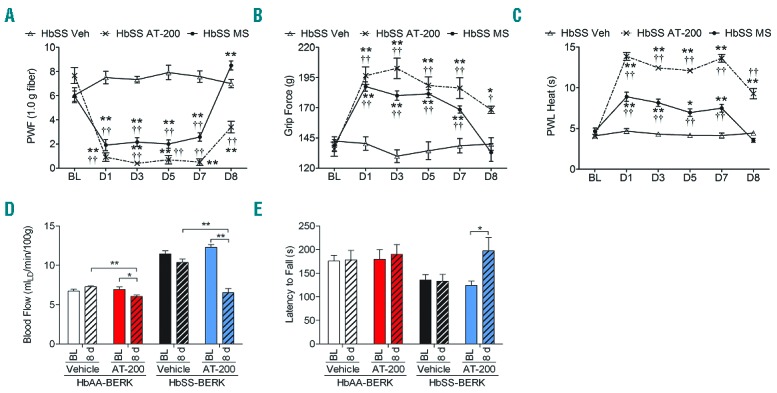
Sustained anti-nociceptive effect and lack of tolerance with chronic AT-200 treatment. Mice were treated daily with vehicle, morphine 20 mg/kg/day or AT-200 10 mg/kg/day for seven days. Sensory testing was performed at baseline before drug treatment and 30 minutes following the drug treatment on days 1, 3, 5 and 7 and on d8 (24 h following the last injection). Responses of sickle mice with each pain stimulus are shown (A–C). A separate set of control and sickle mice were treated daily for seven days with AT-200 or vehicle and tested before (baseline) and after eight days of starting the treatments, i.e. 24 h after the last daily drug injection (given on day 7, for dorsal cutaneous blood flow measured by laser Doppler velocimetry (D) and performance on an accelerating rotarod (0–72 rpm) over a period of 5 min to measure balance and motor co-ordination (E). Statistical significance was calculated by comparing each value to BL (*), and with vehicle (†) for each time point. *P<0.05 and **P<0.005 compared to BL; and ††P<0.005 compared to vehicle for that time point. Mean age of mice ± SEM in months were, HbSS-BERK Vehicle, 4.53 ± 0.22 (n=6); HbSS-BERK Morphine, 4.6± 0.25 and HbSS-BERK AT-200 (n=6), 4.6 ± 0.20. For the analysis of physiological parameters 5 HbAA-BERK mice and 8 HbSS-BERK mice were used in each group. Veh: vehicle; PWF: paw withdrawal frequency; PWL: paw withdrawal latency; BL: baseline; MS: morphine sulphate.
