Abstract
The ovarian hormones progesterone and estrogen play important roles in breast cancer etiology, proliferation, and treatment. Androgens may also contribute to breast cancer risk and progression. In recent years, significant advances have been made in defining the roles of these steroid hormones in stem cell homeostasis in the breast. Stem cells are potential origins of breast cancer and may dictate tumor phenotype. At least a portion of breast cancers are proposed to be driven by cancer stem cells (CSCs), cells that mimic the self-renewing and repopulating properties of normal stem cells, and can confer drug resistance. Progesterone has been identified as the critical hormone regulating normal murine mammary stem cell (MaSC) populations and normal human breast stem cells. Synthetic progestins increase human breast cancer risk; one theory speculates that this occurs through increased stem cells. Progesterone treatment also increases breast CSCs in established breast cancer cell lines. This is mediated in part through progesterone regulation of transcription factors, signal transduction pathways, and microRNAs. There is also emerging evidence that estrogens and androgens can regulate breast CSC numbers. The evolving concept that a breast CSC phenotype is dynamic and can be influenced by cell signaling and external cues emphasizes that steroid hormones could be crucial players in controlling CSC number and function. Here we review recent studies on steroid hormone regulation of breast CSCs, and discuss mechanisms by which this occurs.
Keywords: Progesterone, Progesterone receptor, Cancer stem cells, Hormone replacement therapy, Breast cancer, Steroid hormone
Ovarian Steroid Hormones and Breast Cancer
Removal of the ovaries was recognized as an effective treatment for breast cancer in the late 19th century [1, 2]. It is now well established that three quarters of breast cancers are hormone dependent, requiring local or systemic estrogens for growth maintenance. As such, endocrine therapies targeting the estrogen signaling axis have remained the cornerstone of breast cancer treatment since the first use of tamoxifen in the 1970s [3]. In addition to perpetuating tumor growth, prolonged exposure to ovarian hormones is an independent risk factor for breast cancer; contributing factors include extended menstrual cycles due to early menarche and/or late menopause, a transient risk tied to pregnancy, the use of oral contraception and/or hormone replacement therapies, and excess hormone production in post-menopausal obesity [4–9]. Although the precise mechanisms of how female hormones increase breast cancer risk remain unknown, most theories have centered on increased proliferation imparted to breast epithelial cells. However, the repopularized concept that normal tissue stem cells may be the origin of many solid tumor malignancies has led to speculation that hormones target a specific population of long-lived cells. The seminal discovery that ovarian hormones, particularly progesterone, expand stem cells in the normal murine mammary gland and human breast [10–12] supports this notion and has led to new implications for hormone involvement in tumorigenesis. Emerging evidence reinforces that hormones influence a stem cell phenotype in established tumors. In this review, we discuss steroid hormone-mediated regulation of breast tumor stem cells, with a particular emphasis on progesterone and progestins.
Breast Cancer Stem Cells
The cancer stem cell (CSC) theory posits that tumors contain a subpopulation of cells that share properties of normal stem cells including self-renewal, tumor initiation, indefinite replicative potential, and the ability to generate differentiated progeny [13]. Importantly, CSCs compared to the bulk tumor cells are proposed to have heightened resistance to conventional chemotherapies due to relative quiescence and elevated expression of multi-drug resistance pumps [14, 15]. Breast CSCs in particular show selective resistance to radio-, chemo- and endocrine therapies [16–19]. The notion of a rare static breast CSC population is challenged by developing evidence that the breast CSC phenotype is not necessarily pre-existing, but can be acquired through cytokine signaling and environmental cues [20–22]. This has important implications for hormone receptor positive breast cancers, where endogenous or exogenous hormone exposure could influence the number and activity of CSCs. In fact, our evolving concept of the CSC theory suggests that a combination of CSCs and environmental and clonal pressures collaborate to shape individual tumor phenotype [23, 24].
Several biomarkers have been identified for breast CSCs, albeit with no clear consensus. The seminal paper by Al-Hajj et al. defined breast CSCs as having the surface marker signature CD44+CD24−/lowEpCAM+ (termed CD44+CD24− hereafter) [25]. Primary breast tumor cells with this signature were able to initiate tumors from small numbers of cells in immune deficient mice [25]. CD44+CD24− cells are more abundant in triple negative breast cancers (TNBC) that lack estrogen receptors (ER, alpha) and progesterone receptors (PR), and are less prevalent (0–5 %) in luminal subtype ER+PR+ breast cancers [19, 26, 27]. Furthermore, within a tumor, CD44+ CD24− cells express low ER and PR mRNA compared to CD44−CD24+ cells [28]. Activity of the enzyme aldehyde dehydrogenase (ALDH) was subsequently defined as a marker of normal breast stem cells and breast tumor initiating cells [29]. The ALDH+ and CD44+CD24− populations are not identical in tumors, but share an overlapping population that has the most potent tumor initiating activity [29]. ALDH+ cells have also been reported as ER negative [29, 30]. However, a recent report finds ALDH+ cells exist in both mesenchymal and luminal-like states, although ER expression was not measured [31]. Our group originally reported that luminal ER+PR+ breast cancers contain a subpopulation of cells that express the epithelial intermediate filament protein cytokeratin 5 (CK5), a marker of normal human breast stem and luminal progenitor cells [32–36]. CK5+ cells, compared to the bulk CK5− tumor cells, are endocrine and chemotherapy resistant, and have enhanced mammosphere-forming and tumor-initiating potential [17, 37, 38]. CK5+ cells generally lack expression of ER and PR [37] and their population partially overlaps with CD44+ cells, though non-identical populations exist [37, 38]. Other biomarkers for breast CSCs have been mentioned in the literature less frequently; we focus our discussions here on these three well-described markers.
Progestins, Progesterone Receptors, and Breast Cancer Stem Cells
PR has been measured in breast cancer since the 1970s with the advent of radio ligand binding assays [39]. The presence of PR portends better prognosis and responsiveness to endocrine therapy, and has generally been thought to be an indicator of functional ER [40]. However, activated PR is detrimental for late stage breast cancers, providing some rationale for dual targeting of ER and PR in advanced tumors [41]. PR has two naturally occurring isoforms transcribed from the same gene, a truncated PRA and a longer PRB form [42]. Both isoforms are generally co-expressed in PR+ cells. However, a higher PRA:PRB ratio signifies less favorable outcome for breast cancer [43, 44]. PR ligands include the ovarian hormone progesterone as well as synthetic progestins such as medroxyprogesterone acetate (MPA).
The unexpected findings of the Women’s Health Initiative and the Million Women Study that combination estrogen/progestin but not estrogen alone increased the risk of invasive breast cancer changed our perception of progestins as predominantly onco-protective hormones [45, 46]. Progestin-mediated proliferative stimuli on the post-menopausal normal breast were originally suspected as causing increased breast cancer risk [47]. However, an alternative theory supposes that progestins expand a transformation sensitive pool of normal stem cells, or activate occult malignant stem cells, accelerating the appearance of ER+PR+ tumors [48, 49]. This aligns with early studies by Charles Huggins et al. establishing that administration of progesterone (but not estrogen alone or estrogen plus progesterone) to female Sprague Dawley intact rats fed a single dose of the mutagen 17, 12-dimethylbenz(a)anthracene (DMBA) greatly accelerated time to mammary tumor formation [50]. It was later shown that PR knockout (PRKO) animals had significantly less DMBA-induced mammary tumors than wild-type animals, suggesting PR are crucial for carcinogen-induced mammary tumor formation in rodents [51]. Thus, it is established that progestins and PR play important roles in rodent mammary tumorigenesis, which could potentially occur through modulation of stem cells. The role of progestins in human breast tumorigenesis is less well established. HRT trials suggest that progestins are tumorigenic in post-menopausal women, although there is some speculation that progestins may be accelerating the growth of existing micro-malignancies [49, 52]. In this review, we discuss the role of progestins in established breast cancers.
Progesterone and synthetic progestins increase populations of phenotypical breast CSCs in ER+PR+ breast cancer cell lines. This was first demonstrated in PR-rich T47D xenograft tumors grown in mice supplemented with estrogen alone or estrogen plus the synthetic progestin MPA; the progestin treated tumors had increased numbers of CK5+ tumor cells [37]. Progesterone induction of CK5+ cells occurs within 24 h in multiple luminal breast cancer cell lines including MCF7, ZR75-1, and BT474, but is most potent in T47D cells; these cells have amplification of the PR gene, and express PR in a non-estrogen-dependent manner [17, 53, 54]. In T47D cells, the post-treatment CK5+ population can be up to 20 % of the total cells. Progesterone treatment also increased the total CD44+ cell population by 8–12 fold, measured by flow cytometry, in luminal breast cancer cell lines [53, 54]. MCF7, BT474, and ZR75 cells require pre-treatment with estrogens to induce PR protein levels prior to assessing progesterone action. In these cell lines, estrogen alone did not increase the CD44+ population, whereas estrogen plus progesterone increased the number of CD44+ cells [53, 54]. These experiments measured only the total CD44+ population; luminal breast cancer cell lines are near ubiquitous for CD24+ cells. A recent paper by Hilton et al. [55] demonstrated that treatment with progesterone or the synthetic progestins ORG2058 or MPA increases the CD44+CD24− population in T47D and HCC1428 breast cancer cells. One recent study reported that progesterone treatment increased the ALDH+ population from 1 to 3.5 % in T47D cells [56]. Thus, there is sufficient evidence that in breast cancer cell lines and cell line-derived xenograft models, progesterone or its synthetic analogs can increase breast CSCs as defined by three common markers. There are some exceptions; BCK4 cells, an ER+PR+ breast cancer cell line isolated from a pleural effusion [57], do not increase CK5 protein levels in response to progesterone (with or without estrogen).1 Also, in our collection of breast cancer patient derived xenografts (PDX) [58], we find tumor lines that are both sensitive and resistant to progestin expansion of CK5+ cells2. Thus, the context in which progestins and PR increase breast CSCs warrants further investigation.
Progesterone-expanded breast cancer cells show functional stem cell properties. Our laboratory has engineered T47D cells with stable integration of the human CK5 promoter linked to GFP, allowing FACS isolation of enriched CK5+ and CK5− fractions [38, 59]. We demonstrated that isolated CK5+ compared to CK5− cells following progesterone treatment have increased mammosphere forming capacity [38]. Progesterone-expanded CK5+ vs. CK5− T47D cells also show increased tumor initiation capacity in vivo with limiting dilution analysis.3 CK5+ cells also produce outgrowths of ER+PR+ cells [37], suggesting they are capable of recapitulating tumor heterogeneity. Taken together, these data support that progesterone increases functional CSC activity.
There is some speculation over whether synthetic progestins impart a higher risk for breast cancer than the natural hormone progesterone during hormone replacement regimens [60]. Studies in breast cancer cells identify that both progesterone and progestins increase CSCs similarly. In PR-rich T47D breast cancer cells, both progesterone and MPA regulate a similar program of genes [61]. Likewise, both hormones expand CK5+ cells in T47D xenograft breast tumors [36], and several progestins increase the CD44+CD24− population in T47D breast cancer cells [55]. As progestins, particularly MPA, can target both PR and AR, studies in new patient-derived tumor models with various PR and AR expression may shed light on their contributions to breast CSC expansion.
Estrogens, Estrogen Receptors, and Breast Cancer Stem Cells
Estrogen receptor alpha (ER) remains the single most important prognostic and predictive factor determining breast cancer treatment and outcomes. Like PR, ER measurement also commenced in the early 1970s via radio ligand binding assay [62]. ER is expressed in ~75 % of breast tumors and indicates candidacy for treatment with therapies that target ER through selective ER modulation or degradation (SERMs and SERDs, respectively) or reduction in estrogen production (aromatase inhibitors, AI). These endocrine therapies remain the cornerstone of breast cancer treatment for most patients [63]. Estrogens are the primary mitogens in hormone-dependent breast cancers, with compensatory proliferative pathways in refractory endocrine resistant tumors [64]. While estrogens are pro-proliferative in breast cancer, ER is also positively associated with luminal differentiation markers such as GATA3, CK18, and MUC1 [65]. This is in contrast to the normal human breast where ER+ luminal cells are quiescent while ER− luminal cells are actively proliferating [66].
Estrogens are proposed to play a permissive role in the expansion of normal murine mammary stem cells by stimulating expression of PR [10, 11]. In breast cancers, several studies have reported that estrogens alone do not increase CD44+ or CK5+ CSCs [38, 53, 54, 67]. There are, however, a few studies that have linked estrogens and ER to increased CD44+CD24− breast cancer cells. One study reported that estrogen treatment of MCF7, T47D, and HCC1428 breast cancer cells increased the CD44+CD24− population up to eight-fold [68]. This was hypothesized to occur through a paracrine feedback mechanism involving estrogen induction and secretion of fibroblast growth factor 9 (FGF9), increased expression of the transcription factor Tbx3 in non-CSCs, and further stimulation of Wnts and FGFs to expand the CSC pool. In ER− breast cancers, Tbx3 expression stabilizes paracrine FGF and Wnt signaling to regulate CSC subpopulations [68].
Several studies have reported that estrogens can influence breast CSCs through both non-genomic signaling and variant ERs. G protein-coupled receptor 30 (GPR30), a seven-transmembrane domain receptor, mediates non-genomic estrogen signaling and is expressed in both ER+ and ER− breast cancer cells and tumors [69, 70]. GPR30 regulates the Hippo signaling pathway through activation of tafazzin (TAZ), a phospholipid transacylase [69]. Using an isogenic derivative of the human immortalized epithelial MCF10A cell line (Ras-transformed MCF-10A-T1k cells), CD44+CD24− cells expressed higher levels of TAZ; knockdown of TAZ in these cells significantly reduced the CD44+CD24− population and mammosphere formation capacity [71]. TAZ is also overexpressed in the CSC fraction of primary breast cancers and has been linked to CSC-mediated metastasis [72]. Knockdown of TAZ decreased chemoresistance and the tumorigenic capacity of primary breast CSCs [72].
The ER variant ER-α36 has been implicated in regulating breast CSCs. ER-α36 lacks the ligand-dependent and –independent transactivation domains (ATF-1 and -2), while retaining the DNA binding, dimerization, and ligand-binding domains [73]. In MCF7 and T47D cells, ER-α36 promoted tamoxifen resistance by increasing the self-renewal capacity of CD44+CD24− cells [74, 75]. Knockdown of ER-α36 in T47D and MCF7 cell lines decreased the overall CD44+CD24− population and blocked tamoxifen- or fulvestrant-mediated increases in CD44+CD24− cells. ER-α36 knockdown also decreased mammosphere formation of the same cells [74, 75]. Knockdown of ER-α36 in the HER2+ breast cancer cell line SKBR3 reduced HER2 expression and the ALDH+ CSC population [76]; HER2 has been implicated as a driver of ALDH+ breast CSCs [77]. These data support a role for variant ER-α36 in regulating breast CSCs in both ER+ and HER2+ER− tumors.
Several reports have cited that SERMS, SERDs, and AIs enrich for cells with a CD44+CD24− or CK5+ phenotype. Tamoxifen, fulvestrant, or estrogen depletion increased the percent of CK5+ cells in T47D cell cultures [17]. In a small cohort of patients undergoing neoadjuvant therapy with the AI exemestane plus tamoxifen, CK5 expression increased in post-compared to pre-therapy tumor biopsies from the same patients [17]. Tumors treated with the AI letrozole were enriched in CD44+CD24− mammosphere forming cells post-therapy [18]. Tamoxifen-resistant MCF7 cells have increased CD44+CD24− and ALDH+ populations compared to the parental line and high levels of Sox2, one of four transcription factors involved in induced pluripotent stem cell production. The development of Tam resistance in these cells is driven by Sox2 activation of Wnt signaling, possibly through expansion of CSC populations [78]. Knockdown of Sox2 or inhibition of Wnt signaling reduced CSC populations, inhibited mammosphere formation, and restored tamoxifen sensitivity [78]. These studies suggest that antagonizing estrogen action in breast cancer cells and tumors increases CSCs. This could occur through selection and expansion of the mostly ER− CSC pool, or upregulation of self-renewal signaling pathways such as Wnt.
Androgens, Androgen Receptors, and Breast CSCs
AR is present in 70–80 % of breast cancers [79–81], making it more commonly expressed than either ER or PR. Overall AR, like ER and PR, is associated with more favorable prognosis and a well differentiated phenotype in breast cancer, although this may be subtype specific. AR is co-expressed with ER in 80–90 % of luminal breast cancers where it generally correlates with better prognosis [79, 82–85]. AR has also been detected as a potential co-regulator for ER, both in previous work using two-hybrid systems [86] and more recently using ChIP and proximity ligation assays (PLA) [87]. AR is also found in ER− breast cancer subtypes including HER2+ER− tumors and a subset of TNBC termed luminal AR [81, 88]. Recent data implicate AR as a compensatory mechanism for breast cancer growth in ER− disease; these findings have led to the development of AR-targeted therapeutic approaches for breast cancer [89–91]. Clinical trials are currently centered on the anti-androgen enzalutamide for treatment of AR+ TNBC or endocrine-refractory ER+ tumors [89, 91].
The contribution of androgens and AR in regulating breast CSCs remains only marginally explored. A recent report cited that dihydrotestosterone (DHT) treatment led to a small increase (1–3 %) in the CK5+ population in MCF7 but not T47D cells [67]; the latter may have nonfunctional AR [92]. This same study demonstrated that glucocorticoids and min-eralocorticoids can also expand the CK5+ subpopulation in luminal breast cancer cell lines [67]. This intuitively makes sense as PR, GR, AR, and the mineralocorticoid receptor (MR) share similar DNA binding consensus sequences. In AR+ TNBC cell lines, AR is important in maintaining the CSC population as knockdown of AR and treatment with the anti-androgen enzalutamide each reduce the ALDH+ population and mammosphere formation [93]. It is therefore also likely that AR can regulate breast CSCs in a context-dependent manner.
Many synthetic progestins have partial affinity for AR and can mimic androgen activity in breast cancer cells, as well as cause androgenic side effects in women taking these drugs [94, 95]. Therefore it is possible that progestin-mediated increases in breast CSCs could occur partially through AR. This could be particularly important in breast neoplasms that are AR+PR− or have a higher AR to PR ratio (AR>PR). Furthermore, in HRT-treated women, synthetic progestins had stronger association with increased breast cancer risk than progesterone [96, 97], implicating dual stimulation of AR and PR could be involved. Under some circumstances, AR blocks ER action in breast cancer cells by binding at ER target genes, and acts as an antiestrogen to inhibit growth [98]. This underscores the complexities of AR action in breast cancer, which are likely to be context-dependent [99]. As crosstalk and cooperative interactions between ER, PR, and AR signaling are unraveled, it may become clear that the balance of the three receptors contributes to the regulation of breast CSCs.
Downstream Signaling Pathways and Transcription Factors that Facilitate the Progesterone-Mediated Increase in Cancer Stem Cells
In breast cancer cells, progesterone treatment leads to downstream increases in multiple transcription factors, growth factors, and other proteins that could contribute to an increase in CSCs (illustrated in Fig. 1). Progesterone potently upregulates genes involved in normal mammary development such as prolactin receptor (PRLR) and Signal Transducer and Activator of Transcription 5A (Stat5a). Progesterone and MPA also upregulate or activate signaling pathways involved in CSC self-renewal in T47D breast cancer cells such as members of the Notch signaling pathway (Notch2 and Jagged 1) [61, 100]. Notch signaling is also elevated in CK5+ compared to CK5− breast cancer cells [101]. Progesterone also activates mitogenic Wnt signaling in human breast cancer cell lines through Wnt1, which leads to epidermal growth factor receptor (EGFR)-mediated downstream activation of Erk1/2 mitogen-activated protein kinase (MAPK) activity [102]. EGFR is upregulated and activated by progesterone in breast cancer [103]. While EGFR has not been directly linked to breast CSCs, it is co-expressed with CK5 in both luminal and basal-like breast cancers [101, 104]. HER2 is a reported driver of ALDH+ CSCs in non-HER2 amplified tumors [77, 105], and although the HER2 gene has not been reported as directly regulated by progestins, activated PRs increase HER2 signaling during tumor progression [106]. These data implicate that several progesterone-activated signaling pathways contribute to breast CSC expansion; precise mechanisms of action require further investigation.
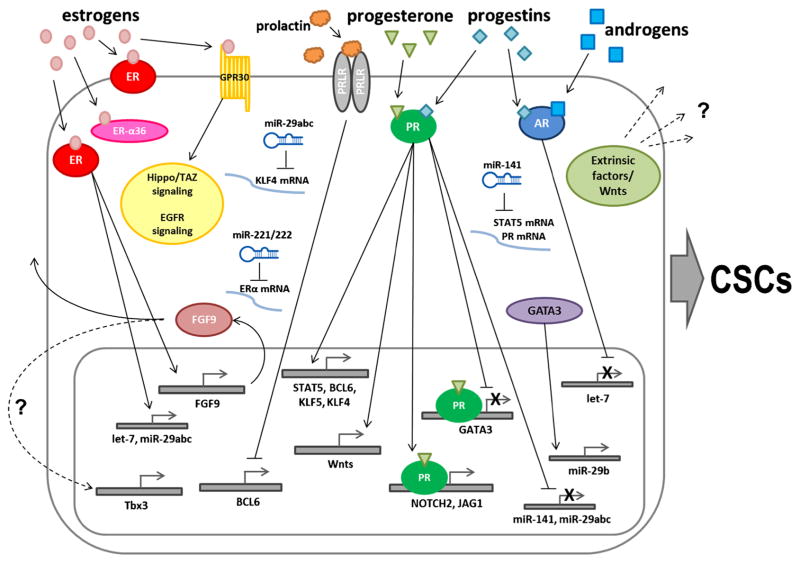
Fig. 1.
Schematic of described mechanisms involved in steroid hormone regulation of cancer stem cell populations. Estrogens bind estrogen receptor alpha (ER) to increase transcription of target genes FGF9, let-7, and miR-29abc. FGF9 is involved in paracrine, and possibly autocrine, signaling to increase transcription of the transcription factor Tbx3. Estrogens can also bind GPR30 to initiate EGFR and Hippo signaling pathways. Progesterone or progestins bind progesterone receptor (PR) to increase expression of transcription factors (Stat5, Bcl6, Klf5, and Klf4), Notch pathway members (Notch2, Jagged1), and members of the Wnt family (Wnt-1). Wnts and other extrinsic factors may be secreted to influence dedifferentiation of the secreting or neighboring cells. Both progesterone and progestins bind PR to downregulate GATA3, miR-141, or miR-29abc, and increase CSC populations; GATA3 upregulates expression of miR-29b. Androgens or progestins bind androgen receptor (AR) to suppress let-7 microRNA transcription. ER downregulates miR-221/222, which represses translation of ER, miR-29abc blocks translation of Klf4, and miR-141 blocks translation of both Stat5 and PR. Prolactin binds prolactin receptor (PRLR) to block expression of the transcription factor BCL6, which suppresses progestin-dependent induction of CK5
Several other transcription factors are reported to positively or negatively influence progesterone/PR− induced CK5 expression. Progesterone-mediated expression of CK5 is preceded by upregulation of BCL6, an oncogene and transcriptional repressor [107]. Prolactin inhibits progesterone-mediated upregulation of CK5 through Stat5a-dependent repression of BCL6 transcription, indicating negative crosstalk between PRLR and PR [107, 108]. This suggests that loss of BCL6 protein may lead to increases in other factors that block PR transcription of the CK5 gene. Krüppel-like factor 5 (KLF5) is another transcription factor upregulated by progesterone in breast cancer cells [61, 100]; knockdown of KLF5 impaired progesterone-mediated induction of CK5, whereas overexpression of KLF5 in the absence of progesterone was able to increase CK5 expression [109]. Progestins also downregulate the transcription factor (and Notch target gene) GATA3, which is associated with maintaining the luminal epithelial phenotype [110]. Using a high content screen, we found that several retinoic acid (RA) compounds were potent inhibitors of progesterone induction of CK5 in T47D breast cancer cells [59]. RA is a strong pro-differentiation hormone in multiple cell types and, contrary to progesterone, can prevent carcinogen-induced mammary tumor formation in rats [111]. Interestingly, RA downregulates PR mRNA and protein levels, and inhibits progestin-stimulated transcription of a PR-regulated reporter construct [112, 113], suggesting negative crosstalk between retinoic acid receptor (RAR) and PR signaling in regulating a breast CSC phenotype. Overall, these studies have identified that several progesterone-regulated transcription factors cooperate in regulating breast CSC populations, while other transcription factors may counterbalance the action of progesterone/PR.
Steroid Hormone-Regulated microRNAs and Breast CSCs
MicroRNAs (miRNAs) are small regulatory RNA molecules that regulate expression of specific target genes by base-pairing to their mRNAs and interfering with translation and/or inducing degradation. Progestins, estrogens, and androgens all regulate miRNAs [114–117], several of which have been linked to CSC formation in breast cancer cells (Fig. 1). The miR-29 and miR-200 families have specifically been studied for their roles in progesterone-induced breast CSC formation. All three miR-29 family members (miR-29abc) are rapidly (within 6 h) downregulated by progestin treatment in breast cancer cells [53]. This downregulation coincides with an increase in protein levels of the transcription factor KLF4, which was found to be a specific target of miR-29a [53]. Inhibition of miR-29a or miR-29b alone was sufficient to increase the CD44+ breast cancer cell population [53, 118] and tumor-initiating ability of breast cancer cells [53]. miR-29a inhibition also potentiated the progestin-mediated increases in both the CD44+ and CK5+ breast cancer cell populations [53]. Progesterone also downregulates GATA3 in breast cancer cells [110], which has been shown to increase miR-29b expression to promote a differentiated phenotype and suppress metastasis [118]; the progestin-induced down-regulation of miR-29b could therefore increase the breast CSC population partially through lowering GATA3.
Progesterone also rapidly downregulates miR-141, a member of the miR-200 family of tumor suppressors [54]. Inhibition of miR-141 also increased the number of CD44+ breast cancer cells, and potentiated progesterone-dependent increases in the CD44+ and CK5+ populations. A combination of miR-141 inhibition plus progesterone treatment increased the tumor initiating capacity of breast cancer cells [54]. MiR-141 was found to directly target both PR and Stat5a mRNA. Knockdown or inhibition of Stat5a using siRNA or the small molecule Pimozide, respectively, reduced the ability of progesterone to increase CK5+ cells [54]. Interestingly, miR-141 as well as other miR-200 family members are underexpressed in CD44+CD24− breast cancer cells [119], suggesting their suppression helps maintain a more stem-like phenotype.
ER-regulated miRNAs in breast cancer cells have been reported by multiple laboratories [114]. While there are no specific studies on ER miRNAs and breast CSCs, ER upregulates several miRNAs involved in maintenance of differentiation including the let-7 miRNA family, proposed to repress self-renewal and promote differentiation in normal and cancer cells [120]. Interestingly, ER upregulates the miR-29 family; this is opposite to the effects of progesterone. One study showed that ER downregulates miR-221/222 [121], a miRNA that represses ER expression [122], and is found in higher levels in CD44+CD24− breast cancer cells [119]. The role of AR-regulated miRNAs in breast CSCs remains unexplored. A few studies have assessed dihydrotestosterone (DHT)-regulated miRNAs in MCF7 and MDA-MB-453 breast cancer cell lines [116, 123, 124]. The majority of miRNAs were downregulated, similar to that described for progestins [124]. AR also downregulates the pro-differentiation miRNA let-7a; overexpression of let-7a inhibited the growth of TNBC cells [123]. Collectively, these studies suggest that ER upregulates miRNAs that maintain a more differentiated phenotype while PR, and perhaps AR, suppress miRNAs that support breast cancer cell differentiation. Further investigation is required to address how hormone regulated miRNAs, and other non-coding RNAs, influence a breast CSC phenotype.
Intrinsic vs Extrinsic Signaling in Progesterone-Mediated Expansion of Breast CSCs
The mechanism of progestin-mediated increases of breast CSCs likely involves both intrinsic and extrinsic signaling. Using a GFP reporter driven by the human CK5 promoter and time-lapse microscopy, we showed that previously CK5− cells become CK5+ upon progesterone treatment [125]. This argues against expansion of pre-existing CK5+ cells through cell division and suggests that progesterone directly stimulates reprogramming from a CK5− to CK5+ state. Interestingly, these CK5+ cells lose expression of ER and PR [38]. To truly test if this occurs in a cell-intrinsic manner will require single cell experimentation. Progestins regulate multiple secreted factors that could act on the producing cell or on neighboring cells to stimulate CK5 expression. Although progestin treatment increases the fraction of cells with breast CSC markers several fold, this still only comprises 5–20 % of the total cell population. Therefore, only specific cells are primed for progesterone reprogramming, implying surrounding cells could contribute via extrinsic signaling but not change phenotype themselves. Altered transcriptomes, pioneer and cofactor expression, and DNA epigenetics in individual cells may all contribute to susceptibility to dedifferentiation. This would be even more pronounced in solid tumors where genetic diversity plays large role in tumor progression. Our knowledge of which cells become CSCs is limited.
Paracrine signaling plays a critical role in progesterone-driven expansion of mammary stem cells (MaSCs) in murine mammary glands [10, 11]. This is mediated through several secreted factors including receptor activator of nuclear factor kappa-B ligand (RANKL), a potent progesterone regulated gene during mammary development and during pregnancy at peak progesterone levels [10]. Progesterone simultaneously increases RANKL in the luminal cell compartment and RANK receptor in the myoepithelial and mammary stem cell compartments [10, 11]. RANKL is also a key paracrine mediator in progestin-dependent mammary tumorigenesis [126, 127]. Along with RANKL, Wnt4 is upregulated by progesterone in the luminal cell compartment [10]; more recently, Wnt4 was identified as a downstream control factor of progesterone-mediated MaSC function [128]. Recent reports have also identified a role for progesterone-mediated CXCR4 paracrine signaling in normal mammary stem and progenitor cells. In normal mouse mammary glands, inhibition of CXCR4 led to a decrease of progesterone-directed expansion of progenitor cell populations and a reduction in colony-forming capacity [129]. Overall, these data implicate a critical role for paracrine signaling in progesterone regulation of murine MaSCs.
Despite the role of signaling in mediating progesterone signals in the murine mammary gland and during mammary tumorigenesis, their role in progesterone signaling in humans is less well studied. RANKL was not a significantly progesterone-regulated gene in genome-wide profiling of human breast organoids [12]. A recent report identified that progesterone increased RANKL in human breast microstructures, but not in isolated HMECs [130]. Furthermore, RANKL expression in human breast specimens was associated with high serum progesterone levels [130]. Despite these observations, multiple studies have not identified RANKL mRNA or protein levels as increased by progestin treatment in PR+ breast cancer cell lines [61, 100, 130–133]. Forced overexpression of RANK and stimulation with RANKL in multiple breast cancer cell lines, however, increased the CD44+CD24− population, as well as increased migration and invasion [133]. Progestins do increase RANKL family members TNFSF10 (TRAIL) and TNSFS10a (TRAIL receptor) [61, 100], and thus could be signaling through a similar system. In normal human breast tissue, cell populations that express growth hormone receptor (GHR) were found to overlap with progenitor cell populations and show some functional properties of stem cells, including the ability to form mammospheres and differentiate into multiple lineages [134]. While these GHR+ cells are hormone receptor negative, progestins act on ER+PR+ cells in the normal breast to induce GH secretion; inhibition of this signaling pathway significantly reduced MCF7 xenograft growth in vivo [134]. In human breast cancer, CXCR4 is linked to poor prognosis and metastasis. While there is no evidence for progestin-mediated regulation of CXCR4 in breast cancer, studies in breast cancer cell lines have shown higher CXCR4 levels in the CD44+CD24− CSC population, and inhibition of CXCR4 decreased mammosphere formation in MCF7 cells [135]. Thus while progesterone signaling appears to switch on a cell-intrinsic ability to transition to CSCs, the involvement of extrinsic factors from neighboring cells cannot be excluded and requires further investigation.
Conclusions
An integral connection between steroid hormones and their cognate receptors in breast tumorigenesis and progression has been established during more than a century of work. More recently, it has been discovered that hormones regulate the balance of stem cell populations in the normal and malignant breast. In the normal breast, it is speculated that increased stem cell populations lead to increased breast cancer susceptibility. It is also speculated that increasing breast CSC populations influences both drug resistance and tumor recurrence. The fact that luminal subtype ER+PR+ tumors can have particularly long latency periods prior to recurrence underscores how increasing long-lived cells even a few fold may contribute to this clinical problem. On the whole, evidence suggests that progestins are the dominant force increasing normal and breast CSC populations, with estrogens playing a more permissive role. The increasing studies of androgens and AR in breast tumor biology are certain to uncover how they contribute to breast CSC levels. Here we have described what is currently known concerning downstream mechanisms of steroid hormone and receptor regulation of breast CSCs. These include direct and indirect regulation of genes, mostly transcription and signal transduction factors, and post-transcriptional regulation of transcription factors through hormone-regulated miRNAs. The progesterone-mediated increase in breast CSCs is likely less dependent on paracrine signaling, as opposed to stem cell upregulation in the normal murine mammary gland. As new inroads into interactions between ER, PR, and AR are made, it is likely that the balance of steroid hormones and their receptors controls heterogeneous populations in breast cancers, and can influence their long term fate.
Acknowledgments
This work was supported in part by grants NIH R01 CA140985 (CAS) and NIH 1F32CA177081 (JFS). The authors wish to apologize to those whose work was not referenced due to space limitations.
Abbreviations
- AR
Androgen receptor
- ALDH
Aldehyde dehydrogenase
- CK5
Cytokeratin 5
- CSC
Cancer stem cell
- DHT
Dihydrotestosterone
- DMBA
7,12-dimethylbenz(a)anthracene
- EGFR
Epidermal growth factor receptor
- ER
Estrogen receptor (alpha)
- FACS
Fluorescent activated cell sorting
- FGF
Fibroblast growth factor
- HRT
Hormone replacement therapy
- MaSC
Mammary stem cell
- MMTV
Mouse mammary tumor virus
- MPA
Medroxyprogesterone acetate
- MUC1
Mucin 1
- PR
Progesterone receptor
- PRKO
Progesterone receptor knockout
- RANK
Receptor activator of nuclear factor kappa-B
- RANKL
receptor activator of nuclear factor kappa-B ligand
- TNBC
triple negative breast cancer
Footnotes
B.M. Jacobsen, personal communication
J. Finlay-Schultz and C.A. Sartorius, unpublished data
J. Finlay-Schultz and C.A. Sartorius, unpublished data
References
- 1.Beatson G. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;148(3803):162–5. doi: 10.1016/S0140-6736(01)72384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd S. On oophorectomy in the treatment of cancer. Br Med J. 1897;2(1918):890–6. doi: 10.1136/bmj.2.1918.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocrinol Relat Cancer. 2014;21(3):R235–46. doi: 10.1530/erc-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 5.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281–91. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):3–15. doi: 10.1023/A:1015714305420. [DOI] [PubMed] [Google Scholar]
- 7.Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6(5):213–8. doi: 10.1186/bcr921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gierisch JM, Coeytaux RR, Urrutia RP, Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1931–43. doi: 10.1158/1055-9965.epi-13-0298. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis. Int J Cancer. 2009;124(3):698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 10.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–7. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 11.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 12.Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, et al. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150(7):3318–26. doi: 10.1210/en.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 14.Elliott A, Adams J, Al-Hajj M. The ABCs of cancer stem cell drug resistance. IDrugs. 2010;13(9):632–5. [PubMed] [Google Scholar]
- 15.Moore N, Lyle S. Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol. 2011 doi: 10.1155/2011/396076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, et al. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2011;128(1):45–55. doi: 10.1007/s10549-010-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108(19):7950–5. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108(4):1397–402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Laterra J. Cancer stem cells: distinct entities or dynamically regulated phenotypes? Cancer Res. 2012;72(3):576–80. doi: 10.1158/0008-5472.can-11-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–96. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 25.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10(3):R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morimoto K, Kim SJ, Tanei T, Shimazu K, Tanji Y, Taguchi T, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100(6):1062–8. doi: 10.1111/j.1349-7006.2009.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2(1):78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15(8):907–13. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 33.Bocker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab Invest. 2002;82(6):737–46. doi: 10.1097/01.LAB.0000017371.72714.C5. [DOI] [PubMed] [Google Scholar]
- 34.Boecker W, Buerger H. Evidence of progenitor cells of glandular and myoepithelial cell lineages in the human adult female breast epithelium: a new progenitor (adult stem) cell concept. Cell Prolif. 2003;36(Suppl 1):73–84. doi: 10.1046/j.1365-2184.36.s.1.7.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177(1):87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartorius CA, Harvell DM, Shen T, Horwitz KB. Progestins initiate a luminal to myoepithelial switch in estrogen-dependent human breast tumors without altering growth. Cancer Res. 2005;65(21):9779–88. doi: 10.1158/0008-5472.can-05-0505. [DOI] [PubMed] [Google Scholar]
- 37.Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A. 2008;105(15):5774–9. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Axlund SD, Yoo BH, Rosen RB, Schaack J, Kabos P, Labarbera DV, et al. Progesterone-inducible cytokeratin 5-positive cells in luminal breast cancer exhibit progenitor properties. Horm Cancer. 2013;4(1):36–49. doi: 10.1007/s12672-012-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horwitz KB, McGuire WL. Specific progesterone receptors in human breast cancer. Steroids. 1975;25(4):497–505. doi: 10.1016/0039-128X(75)90027-6. [DOI] [PubMed] [Google Scholar]
- 40.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–35. doi: 10.1200/jco.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Knutson TP, Lange CA. Tracking progesterone receptor-mediated actions in breast cancer. Pharmacol Ther. 2013;142(1):114–25. doi: 10.1016/j.pharmthera.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9(5):1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72(2):163–72. doi: 10.1023/A:1014820500738. [DOI] [PubMed] [Google Scholar]
- 44.Graham JD, Yeates C, Balleine RL, Harvey SS, Milliken JS, Bilous AM, et al. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995;55(21):5063–8. [PubMed] [Google Scholar]
- 45.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–92. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood CE, Branstetter D, Jacob AP, Cline JM, Register TC, Rohrbach K, et al. Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res. 2013;15(4):R62. doi: 10.1186/bcr3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narod SA. Hormone replacement therapy and the risk of breast cancer. Nat Rev Clin Oncol. 2011;8(11):669–76. doi: 10.1038/nrclinonc.2011.110. [DOI] [PubMed] [Google Scholar]
- 49.Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab. 2008;93(9):3295–8. doi: 10.1210/jc.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huggins C, Moon RC, Morii S. Extinction of experimental mammary cancer. I. Estradiol-17beta and progesterone. Proc Natl Acad Sci U S A. 1962;48:379–86. doi: 10.1073/pnas.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lydon JP, Ge G, Kittrell FS, Medina D, O’Malley BW. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999;59(17):4276–84. [PubMed] [Google Scholar]
- 52.Santen RJ, Song Y, Yue W, Wang JP, Heitjan DF. Effects of menopausal hormonal therapy on occult breast tumors. J Steroid Biochem Mol Biol. 2013;137:150–6. doi: 10.1016/j.jsbmb.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, et al. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene. 2013;32(20):2555–64. doi: 10.1038/onc.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finlay-Schultz J, Cittelly DM, Hendricks P, Patel P, Kabos P, Jacobsen BM, et al. Progesterone downregulation of miR-141 contributes to expansion of stem-like breast cancer cells through maintenance of progesterone receptor and Stat5a. Oncogene. 2014 doi: 10.1038/onc.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hilton HN, Santucci N, Silvestri A, Kantimm S, Huschtscha LI, Graham JD, et al. Progesterone stimulates progenitor cells in normal human breast and breast cancer cells. Breast Cancer Res Treat. 2014 doi: 10.1007/s10549-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 56.Vares G, Cui X, Wang B, Nakajima T, Nenoi M. Generation of breast cancer stem cells by steroid hormones in irradiated human mammary cell lines. PLoS One. 2013;8(10):e77124. doi: 10.1371/journal.pone.0077124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jambal P, Badtke MM, Harrell JC, Borges VF, Post MD, Sollender GE, et al. Estrogen switches pure mucinous breast cancer to invasive lobular carcinoma with mucinous features. Breast Cancer Res Treat. 2013;137(2):431–48. doi: 10.1007/s10549-012-2377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabos P, Finlay-Schultz J, Li C, Kline E, Finlayson C, Wisell J, et al. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat. 2012;135(2):415–32. doi: 10.1007/s10549-012-2164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoo BH, Axlund SD, Kabos P, Reid BG, Schaack J, Sartorius CA, et al. A high-content assay to identify small-molecule modulators of a cancer stem cell population in luminal breast cancer. J Biomol Screen. 2012;17(9):1211–20. doi: 10.1177/1087057112452138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103–11. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghatge RP, Jacobsen BM, Schittone SA, Horwitz KB. The progestational and androgenic properties of medroxyprogesterone acetate: gene regulatory overlap with dihydrotestosterone in breast cancer cells. Breast Cancer Res. 2005;7(6):R1036–50. doi: 10.1186/bcr1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeSombre ER, Smith S, Block GE, Ferguson DJ, Jensen EV. Prediction of breast cancer response to endocrine therapy. Cancer Chemother Rep. 1974;58(4):513–9. [PubMed] [Google Scholar]
- 63.Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23(8):1616–22. doi: 10.1200/jco.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 64.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 66.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57(22):4987–91. [PubMed] [Google Scholar]
- 67.Goodman CR, Sato T, Peck AR, Girondo MA, Yang N, Liu C, et al. Steroid induction of therapy-resistant cytokeratin-5-positive cells in estrogen receptor-positive breast cancer through a BCL6-dependent mechanism. Oncogene. 2015 doi: 10.1038/onc.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci U S A. 2010;107(50):21737–42. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125(5):2123–35. doi: 10.1172/jci79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei W, Chen ZJ, Zhang KS, Yang XL, Wu YM, Chen XH, et al. The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Dis. 2014;5:e1428. doi: 10.1038/cddis.2014.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–72. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 72.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34(6):681–90. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 73.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336(4):1023–7. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 74.Deng H, Yin L, Zhang XT, Liu LJ, Wang ML, Wang ZY. ER-alpha variant ER-alpha36 mediates antiestrogen resistance in ER-positive breast cancer stem/progenitor cells. J Steroid Biochem Mol Biol. 2014;144(Pt B):417–26. doi: 10.1016/j.jsbmb.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 75.Deng H, Zhang XT, Wang ML, Zheng HY, Liu LJ, Wang ZY. ER-alpha36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PLoS One. 2014;9(2):e88034. doi: 10.1371/journal.pone.0088034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang L, Guo Y, Zhang X, Meng J, Wang ZY. A positive cross-regulation of HER2 and ER-alpha36 controls ALDH1 positive breast cancer cells. J Steroid Biochem Mol Biol. 2011;127(3–5):262–8. doi: 10.1016/j.jsbmb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ithimakin S, Day KC, Malik F, Zen Q, Dawsey SJ, Bersano-Begey TF, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res. 2013;73(5):1635–46. doi: 10.1158/0008-5472.can-12-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piva M, Domenici G, Iriondo O, Rabano M, Simoes BM, Comaills V, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2014;6(1):66–79. doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24(7):924–31. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23(2):205–12. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 81.Guedj M, Marisa L, de Reynies A, Orsetti B, Schiappa R, Bibeau F, et al. A refined molecular taxonomy of breast cancer. Oncogene. 2012;31(9):1196–206. doi: 10.1038/onc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park S, Koo JS, Kim MS, Park HS, Lee JS, Kim SI, et al. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22(8):1755–62. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- 83.Tsang JY, Ni YB, Chan SK, Shao MM, Law BK, Tan PH, et al. Androgen receptor expression shows distinctive significance in ER positive and negative breast cancers. Ann Surg Oncol. 2014;21(7):2218–28. doi: 10.1245/s10434-014-3629-2. [DOI] [PubMed] [Google Scholar]
- 84.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17(7):1867–74. doi: 10.1158/1078-0432.ccr-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, et al. The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer. 1996;32A(9):1560–5. doi: 10.1016/0959-8049(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 86.Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000;167(1–2):139–50. doi: 10.1016/S0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 87.Rechoum Y, Rovito D, Iacopetta D, Barone I, Ando S, Weigel NL, et al. AR collaborates with ERalpha in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat. 2014 doi: 10.1007/s10549-014-3082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011 doi: 10.1172/jci45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16(1):R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015 doi: 10.1158/1535-7163.mct-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chia K, O’Brien M, Brown M, Lim E. Targeting the androgen receptor in breast cancer. Curr Oncol Rep. 2015;17(2):427. doi: 10.1007/s11912-014-0427-8. [DOI] [PubMed] [Google Scholar]
- 92.Nordeen SK, Kuhnel B, Lawler-Heavner J, Barber DA, Edwards DP. A quantitative comparison of dual control of a hormone response element by progestins and glucocorticoids in the same cell line. Mol Endocrinol. 1989;3(8):1270–8. doi: 10.1210/mend-3-8-1270. [DOI] [PubMed] [Google Scholar]
- 93.Barton VN, Richer JK. Androgen Receptor Biology in Triple Negative Breast Cancer: a Case for Classification as AR+ or Quadruple Negative Disease. 2015 doi: 10.1007/s12672-015-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol Cell Endocrinol. 1999;154(1–2):11–20. doi: 10.1016/S0303-7207(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 95.Bullock LP, Bardin CW, Sherman MR. Androgenic, antiandrogenic, and synandrogenic actions of progestins: role of steric and allosteric interactions with androgen receptors. Endocrinology. 1978;103(5):1768–82. doi: 10.1210/endo-103-5-1768. [DOI] [PubMed] [Google Scholar]
- 96.Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448–54. doi: 10.1002/ijc.20710. [DOI] [PubMed] [Google Scholar]
- 97.Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–8. doi: 10.1200/jco.2007.13.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69(15):6131–40. doi: 10.1158/0008-5472.can-09-0452. [DOI] [PubMed] [Google Scholar]
- 99.McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of androgen receptor signalling in breast cancer. Endocrinol Relat Cancer. 2014;21(4):T161–81. doi: 10.1530/erc-14-0243. [DOI] [PubMed] [Google Scholar]
- 100.Clarke CL, Graham JD. Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PLoS One. 2012;7(4):e35859. doi: 10.1371/journal.pone.0035859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haughian JM, Pinto MP, Harrell JC, Bliesner BS, Joensuu KM, Dye WW, et al. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc Natl Acad Sci U S A. 2012;109(8):2742–7. doi: 10.1073/pnas.1106509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27(2):466–80. doi: 10.1128/mcb.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72(2):188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–76. doi: 10.1158/1078-0432.ccr-07-1658. [DOI] [PubMed] [Google Scholar]
- 105.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27(47):6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, et al. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012;14(3):R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sato T, Tran TH, Peck AR, Girondo MA, Liu C, Goodman CR, et al. Prolactin suppresses a progestin-induced CK5-positive cell population in luminal breast cancer through inhibition of progestin-driven BCL6 expression. Oncogene. 2014;33(17):2215–24. doi: 10.1038/onc.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A, et al. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res. 2010;70(4):1711–21. doi: 10.1158/0008-5472.can-09-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu R, Zhou Z, Zhao D, Chen C. The induction of KLF5 transcription factor by progesterone contributes to progesterone-induced breast cancer cell proliferation and dedifferentiation. Mol Endocrinol. 2011;25(7):1137–44. doi: 10.1210/me.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Izzo F, Mercogliano F, Venturutti L, Tkach M, Inurrigarro G, Schillaci R, et al. Progesterone receptor activation downregulates GATA3 by transcriptional repression and increased protein turnover promoting breast tumor growth. Breast Cancer Res. 2014;16(6):491. doi: 10.1186/s13058-014-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anzano MA, Byers SW, Smith JM, Peer CW, Mullen LT, Brown CC, et al. Prevention of breast cancer in the rat with 9-cis-retinoic acid as a single agent and in combination with tamoxifen. Cancer Res. 1994;54(17):4614–7. [PubMed] [Google Scholar]
- 112.Clarke CL, Graham J, Roman SD, Sutherland RL. Direct transcriptional regulation of the progesterone receptor by retinoic acid diminishes progestin responsiveness in the breast cancer cell line T-47D. J Biol Chem. 1991;266(28):18969–75. [PubMed] [Google Scholar]
- 113.Clarke CL, Roman SD, Graham J, Koga M, Sutherland RL. Progesterone receptor regulation by retinoic acid in the human breast cancer cell line T-47D. J Biol Chem. 1990;265(21):12694–700. [PubMed] [Google Scholar]
- 114.Klinge CM. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol Cell Endocrinol. 2015 doi: 10.1016/j.mce.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cochrane DR, Jacobsen BM, Connaghan KD, Howe EN, Bain DL, Richer JK. Progestin regulated miRNAs that mediate progesterone receptor action in breast cancer. Mol Cell Endocrinol. 2012;355(1):15–24. doi: 10.1016/j.mce.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tessel MA, Krett NL, Rosen ST. Steroid receptor and microRNA regulation in cancer. Curr Opin Oncol. 2010;22(6):592–7. doi: 10.1097/CCO.0b013e32833ea80c. [DOI] [PubMed] [Google Scholar]
- 117.Fletcher CE, Dart DA, Bevan CL. Interplay between steroid signalling and microRNAs: implications for hormone-dependent cancers. Endocrinol Relat Cancer. 2014;21(5):R409–29. doi: 10.1530/erc-14-0208. [DOI] [PubMed] [Google Scholar]
- 118.Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell Biol. 2013;15(2):201–13. doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bussing I, Slack FJ, Grosshans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14(9):400–9. doi: 10.1016/j.molmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 121.Di Leva G, Gasparini P, Piovan C, Ngankeu A, Garofalo M, Taccioli C, et al. MicroRNA cluster 221–222 and estrogen receptor alpha interactions in breast cancer. J Natl Cancer Inst. 2010;102(10):706–21. doi: 10.1093/jnci/djq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cochrane DR, Howe EN, Spoelstra NS, Richer JK. Loss of miR-200c: a marker of aggressiveness and chemoresistance in female reproductive cancers. J Oncol. 2010;2010:821717. doi: 10.1155/2010/821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lyu S, Yu Q, Ying G, Wang S, Wang Y, Zhang J, et al. Androgen receptor decreases CMYC and KRAS expression by upregulating let-7a expression in ER-, PR-, AR+ breast cancer. Int J Oncol. 2014;44(1):229–37. doi: 10.3892/ijo.2013.2151. [DOI] [PubMed] [Google Scholar]
- 124.Nakano K, Miki Y, Hata S, Ebata A, Takagi K, McNamara KM, et al. Identification of androgen-responsive microRNAs and androgen-related genes in breast cancer. Anticancer Res. 2013;33(11):4811–9. [PubMed] [Google Scholar]
- 125.Reid BG, Jerjian T, Patel P, Zhou Q, Yoo BH, Kabos P, et al. Live multicellular tumor spheroid models for high-content imaging and screening in cancer drug discovery. Curr Chem Genomics Transl Med. 2014;8(Suppl 1):27–35. doi: 10.2174/2213988501408010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468(7320):98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–7. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 128.Rajaram RD, Buric D, Caikovski M, Ayyanan A, Rougemont J, Shan J, et al. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34(5):641–52. doi: 10.15252/embj.201490434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shiah YJ, Tharmapalan P, Casey AE, Joshi PA, McKee TD, Jackson HW, et al. A progesterone-CXCR4 axis controls mammary progenitor cell fate in the adult gland. Stem Cell Rep. 2015;4(3):313–22. doi: 10.1016/j.stemcr.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med. 2013;5(182):182ra55. doi: 10.1126/scitranslmed.3005654. [DOI] [PubMed] [Google Scholar]
- 131.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–18. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 132.Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol Endocrinol. 2005;19(3):574–87. doi: 10.1210/me.2004-0287. [DOI] [PubMed] [Google Scholar]
- 133.Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012;72(11):2879–88. doi: 10.1158/0008-5472.can-12-0044. [DOI] [PubMed] [Google Scholar]
- 134.Lombardi S, Honeth G, Ginestier C, Shinomiya I, Marlow R, Buchupalli B, et al. Growth hormone is secreted by normal breast epithelium upon progesterone stimulation and increases proliferation of stem/progenitor cells. Stem Cell Rep. 2014;2(6):780–93. doi: 10.1016/j.stemcr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ablett MP, O’Brien CS, Sims AH, Farnie G, Clarke RB. A differential role for CXCR4 in the regulation of normal versus malignant breast stem cell activity. Oncotarget. 2014;5(3):599–612. doi: 10.18632/oncotarget.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]