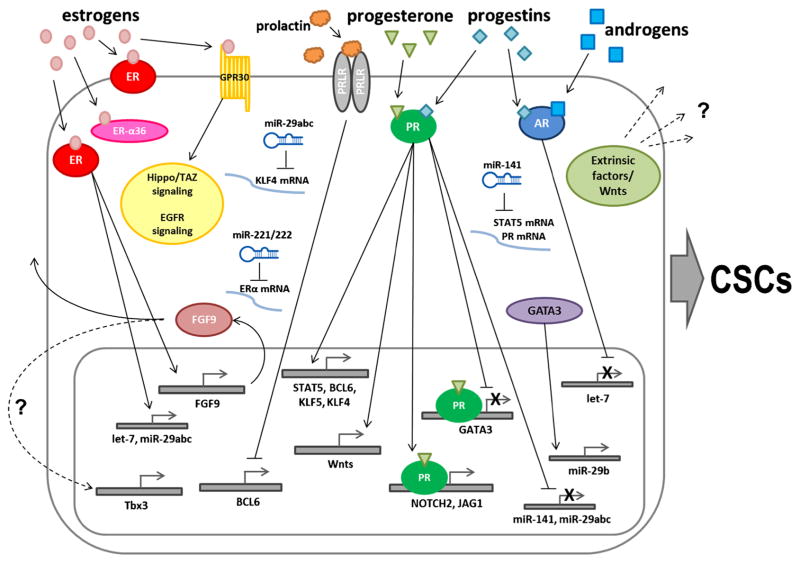
Fig. 1.
Schematic of described mechanisms involved in steroid hormone regulation of cancer stem cell populations. Estrogens bind estrogen receptor alpha (ER) to increase transcription of target genes FGF9, let-7, and miR-29abc. FGF9 is involved in paracrine, and possibly autocrine, signaling to increase transcription of the transcription factor Tbx3. Estrogens can also bind GPR30 to initiate EGFR and Hippo signaling pathways. Progesterone or progestins bind progesterone receptor (PR) to increase expression of transcription factors (Stat5, Bcl6, Klf5, and Klf4), Notch pathway members (Notch2, Jagged1), and members of the Wnt family (Wnt-1). Wnts and other extrinsic factors may be secreted to influence dedifferentiation of the secreting or neighboring cells. Both progesterone and progestins bind PR to downregulate GATA3, miR-141, or miR-29abc, and increase CSC populations; GATA3 upregulates expression of miR-29b. Androgens or progestins bind androgen receptor (AR) to suppress let-7 microRNA transcription. ER downregulates miR-221/222, which represses translation of ER, miR-29abc blocks translation of Klf4, and miR-141 blocks translation of both Stat5 and PR. Prolactin binds prolactin receptor (PRLR) to block expression of the transcription factor BCL6, which suppresses progestin-dependent induction of CK5