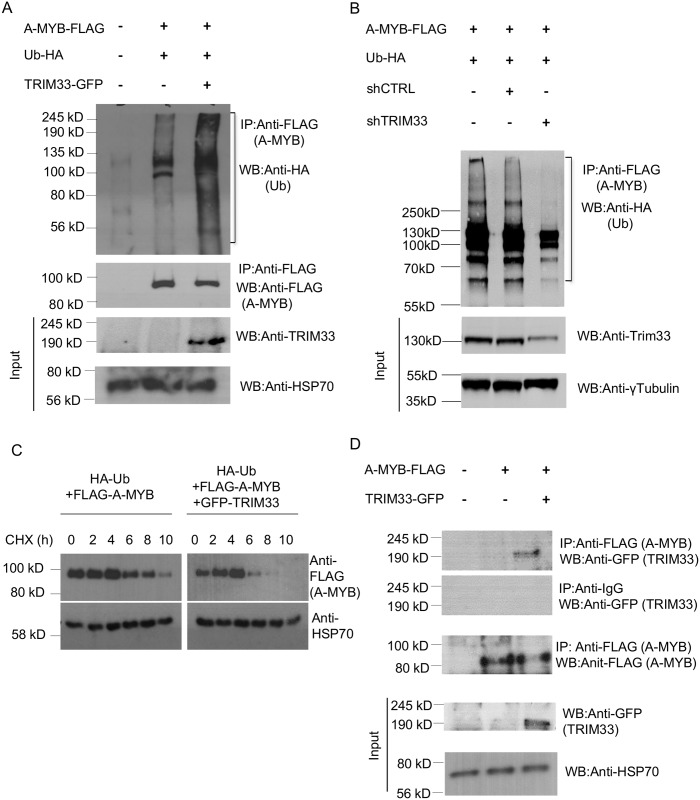
Fig 4. TRIM33 regulates A-MYB stability through ubiquitination.
(A) HEK293T cells were transfected with HA-tagged ubiquitin and FLAG-tagged A-MYB in the presence or in the absence of GFP-tagged TRIM33. To detect ubiquitinated A-MYB, immunoprecipitation was performed on the lysates using anti-FLAG affinity beads and a Western blot was performed using anti-HA antibodies (top panel). To detect the immunoprecipitated A-MYB, the Western blot was probed with anti-FLAG antibodies (middle panel). Expression of tagged TRIM33 and the loading control HSP70 is shown in the bottom panel. (B) HEK293T cells were transfected with HA-tagged ubiquitin and FLAG-tagged A-MYB and either a control or TRIM33 shRNA expression vector. A-MYB was immunoprecipitated and Western blotting was performed as in (A) to detect ubiquitinated A-MYB (top panel). Lysates were subjected to Western blotting with anti-TRIM33 antibody to show knock down and anti-γ-Tubulin antibody as a loading control (bottom panels). (C) The protein stability of A-MYB in the presence and in the absence of TRIM33 was monitored using the protein translation inhibitor cycloheximide (CHX). Cells were transfected as in (A) and CHX was added at 10 μg/ml and samples were analyzed by Western blots at various time points, as shown. HSP70 was used as the loading control. (D) FLAG-tagged A-MYB and GFP-tagged TRIM33 were co-expressed in HEK293T cells. Lysates were subjected to immunoprecipitation with anti-FLAG beads or, as a control, anti-IgG beads, and co-immunoprecipitation of TRIM33 with A-MYB was detected by Western blotting with anti-GFP and anti-FLAG antibodies, respectively (top panels). Expression of tagged Trim33 in lysate is shown by Western blotting with anti-GFP antibody and as a loading control anti-HSP70 is also shown (bottom panels).