Abstract
The ookinete is the motile form of the malaria parasite that invades the mosquito midgut epithelium to initiate sporogony. Differentiation of ingested gametocytes into ookinetes in the mosquito midgut lumen and the subsequent interaction with the luminal surface of the midgut epithelium in preparation for invasion are complex processes. To facilitate the study of these events in detail, it is necessary to produce sufficient numbers of pure, fully mature ookinetes. However, production of even a small number of Plasmodium falciparum ookinetes in vitro has proven to be a daunting task. Consequently, over the past four decades our collective understanding of the biology of this parasite form remains sorely deficient. Here, we describe a new culture technique, which improves the in vitro transformation efficiency of P. falciparum gametocytes into mature ookinetes and supports the complete development of ookinetes that retain the ability to infect the mosquito midgut and to produce oocysts.
Keywords: P. falciparum gametocytes, Ookinetes, Gametocytes to ookinete transformation
1. Introduction
Soon after the mosquito ingests a Plasmodium-infected blood meal, Plasmodium gametocytes differentiate into gametes that mate to form zygotes and later motile ookinetes. To exit the mosquito midgut lumen, ookinetes traverse the midgut epithelium and lodge beneath the basal lamina where they differentiate into oocysts. Upon maturation, each oocyst releases several thousand sporozoites into the hemocoel from where they invade the salivary glands. At this point, the sporozoites are ready to be transmitted to a new vertebrate host when the mosquito takes another blood meal (1). Very little is known about the developmental processes that operate during the differentiation of gametocytes into ookinetes (2).
While gametocytes can be obtained from an in vitro P. falciparum culture, typical methods for the transformation of gametocytes into ookinetes are poorly efficient with a reported transformation efficiency of only 0.002% (0.2 mature ookinete per 10,000 RBC) (3). This is in contrast with the in vitro differentiation of the rodent parasite P. berghei, which is efficient and yields about 106 ookinetes from a single infected mouse (4). Progress has been made recently to formulate media to improve the in vitro production of mature P. falciparum (5–7) and P. vivax (8) ookinetes. Here, we describe a culture medium that supports the efficient differentiation and development of mature P. falciparum ookinetes. We use 20% human serum instead of 20% fetal bovine serum (FBS) (3) and add human RBC lysates and mosquito pupal extracts as supplements.
2. Materials
2.1. Media, Serum and Chemicals
Media: RPMI 1640 (Invitrogen, cat. no.11875-093), Schneiders medium (Invitrogen, cat. no. R690-07), Waymouth medium (Invitrogen, cat. no. 11220-035).
Serum: Human serum (O positive, Interstate Blood Bank, Memphis, TN).
Hypoxanthine (Sigma, cat. no. H9636).
Giemsa stain (Sigma, cat. no. GS500, M7011).
Sodium bicarbonate (Fisher, cat. no. S495-500).
Malaria gas mixture (5% CO2, 5% O2, and 90% N2) (Air Gas, Puritan Medical, cat. no. Z03-NI9022000033).
2.2. Parasites
Plasmodium falciparum, NF54 isolate.
2.3. Equipment
Laminar flow hood (safety cabinet, Baker Co. sterile GARD III advance).
Tabletop centrifuge (Eppendorf, 5810).
Humidified tissue culture incubator (Barnstead Lab-Line, Model305).
Shaker (Kika-Werke Gmbh and Co. D79219).
Microscope with digital camera (Leica, DMLB).
Dounce homogenizer, 7 ml Bellco Glass Inc (cat. no. NC9693819).
Coplin Jar for Giemsa staining (Fisher Scientific).
3. Methods
3.1. Parasite Culture
Prepare P. falciparum culture medium: RPMI 1640 medium containing L-glutamine supplemented with 0.2% sodium bicarbonate and 10% (v/v) human serum.
Wash the human RBC three times with RPMI 1640 medium, resuspend to 50% hematocrit, and store at 4°C.
Maintain NF54 P. falciparum parasites in culture according to standard methods (37°C chamber and malaria gas mixture) while maintaining a 5% hematocrit in the P. falciparum culture medium (9, 10) (see Note 1).
3.2. Induction of Gametocyte Differentiation
When parasitemia reaches about 2–3%, stop adding fresh human blood and induce gametocytemia by not providing additional RBC and changing the medium every day until day 18 (see Note 2).
3.3. Preparation of An. gambiae Pupal Extract
Collect about 500 An. gambiae pupae, wash with sterile distilled water several times and with PBS in the final wash, and store at −80°C until use.
Thaw frozen pupae, resuspend in 500 μl of PBS, and transfer to a glass homogenizer.
Homogenize on ice with 10–12 strokes and centrifuge at 14,500 × g for 10 min at 4°C.
Transfer the supernatant into a clean tube.
Heat the clarified supernatant at 60°C for 1 h and cool to room temperature (RT).
Centrifuge at 14,500 × g for 30 min at 4°C and store the clear supernatant at −80°C until use (see Note 3).
3.4. Preparation of Human RBC Lysate
Wash human, O-positive RBC (about 0.5 ml of packed volume) in sterile PBS.
Add 900 μl of sterile distilled water and swirl for 30 s to lyse the RBC.
Immediately following lysis, add 100 μl of 10× PBS to the tube to make it isotonic.
Centrifuge the lysate at 2,500 × g for 30 min at 4°C, aliquot the supernatant, and store at −80°C until use (see Note 4).
3.5. Preparation of Ookinete Medium
Prepare a 100-ml stock ookinete medium by mixing 22.6 ml RPMI 1640, 22.6 ml Schneiders medium, 22.6 ml Waymouth medium, 20 ml human serum, 1 ml 50 μM hypoxanthine, 5 ml 4% sodium bicarbonate, 4 ml human RBC lysates, and 2 ml pupal extracts. Adjust pH to 7.4 with 10 N NaOH.
Filter-sterilize through a Corning filter sterilization unit (see Note 5).
3.6. Giemsa Staining
Dilute the Giemsa stain with tap water (1:10 dilution) in a conical tube and mix well by inverting the tube for three to five times.
Pour it into a Coplin Jar, place the slides in between the grooves in an upright position, and leave for 3–5 min at RT.
Wash the slides with running tap water for 2–3 min, air-dry and store at RT (see Note 6).
3.7. Determination of Gametocytemia
On alternate days, take about 10 μl of the culture and make a smear on a slide, fix with methanol, and stain with Giemsa (1:10) to count the percentage of gametocytes (see Note 7).
3.8. Measuring Exflagellation
Take 100 μl of the gametocyte culture on day 18, centrifuge at 650 × g for 5 min and resuspend the packed cells in the same volume of ookinete medium.
Drop 4–6 μl of the gametocyte culture onto a slide. Breathe on the coverslip before placing it over the droplet.
Incubate the slide at RT to induce exflagellation (~10–15 min). After 10 min, count the number of exflagellation events using a 40× objective. If no exflagellation event is observed after 10 min, check every 2 min until exflagellation is observed to begin assessment (see Note 8).
3.9. In Vitro Transformation to Produce Ookinetes
After checking gametocytemia (2–3%), transfer 3 ml of the culture material into a 15-ml conical tube and centrifuge at 650 × g for 5 min at 37°C.
Gently resuspend the packed cells in 250 μl of ookinete culture medium.
Transfer into one well of a 24-well tissue culture plate and incubate at 24 ± 2°C on a shaker (50 revolutions per min) for 24 h (see Note 9).
3.10. Preparation of Ookinete Slides
Centrifuge 100 μl of the gametocyte culture at 200 × g for 5 min and discard the supernatant.
Resuspend the packed cells in 20 μl of human serum by flicking the tube and make a thin smear of the mixture on two to four slides.
Air-dry the slides and fix with absolute methanol for 2–3 min at RT. Store all slides at RT for subsequent histological analysis by Giemsa staining using the same method as for gametocyte staining (see Fig. 1 and Note 10).
Fig. 1.
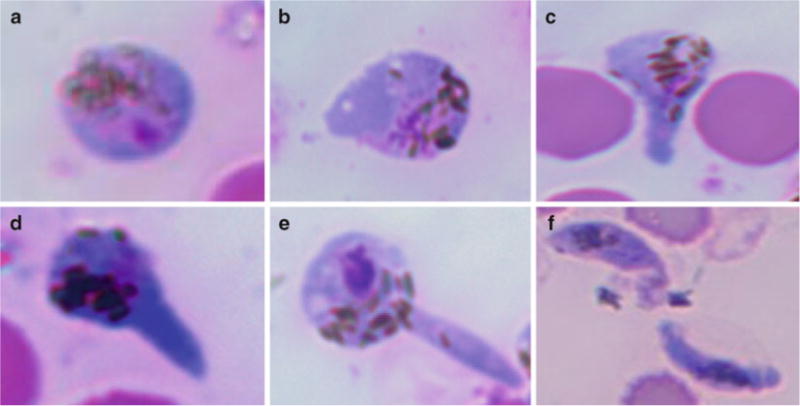
Stages of ookinete differentiation. (a) Zygote after 6 h of culture. (b–f) Stages 1–5, respectively (adapted from ref. 6).
3.11. Ookinete Counting
Count the different stages of ookinete differentiation by microscopic examination of the Giemsa-stained slides with a 100× oil immersion objective. Determine the total number of each ookinete stage per 5,000 RBC (see Fig. 2 and Note 11).
Fig. 2.
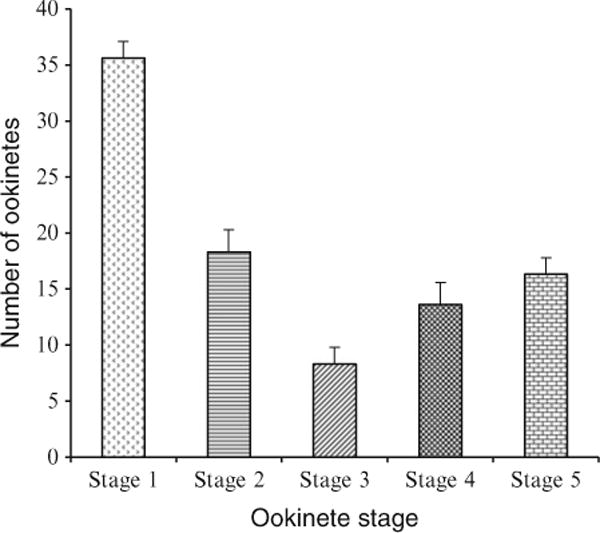
Number of different ookinete stages per 10,000 RBC after 24 h incubation in ookinete medium. Mean of three independent experiments (from ref. 6).
3.12. Photography
Capture the images using a CCD camera and appropriate software (e.g., Spot 3 software) under oil immersion using a 100 × g objective.
Analyze image quality and merge in ADOBE Photoshop 7 (see Note 12).
Acknowledgments
This work was supported by grants from the National Institutes of Health, by the Johns Hopkins Malaria Research Institute and by the Bloomberg Family Foundation.
Footnotes
For P. falciparum culture, blood group is critical and should be O-positive. Human blood and serum need to be handled carefully in a laminar flow hood under aseptic conditions.
The medium of the gametocyte culture needs to be changed daily because accumulation of metabolites is detrimental. For changing the medium, use a sterile Pasteur pipette connected to a vacuum line and suck the medium from the upper level of the culture well, being careful not to disturb the bottom layer containing the infected RBC.
The pupal extract contains xanthurenic acid, a gametocyte-activating agent found in mosquito eyes and midgut (11, 12). The heat treatment is critical and should be 55–60°C for 1 h. Higher temperature could degrade the gametocyte activating factor (xanthurenic acid).
Immediately after lysis of the RBC, it is important to readjust the salt concentration to avoid protein aggregation.
The pH of the transformation medium is critical. It is better to store the medium in a −20°C freezer in 10-ml or 20-ml aliquots. An aliquot of medium should be thawed at RT on the day of transformation and readjusted to pH 7.6.
The Coplin Jar is useful to avoid deposition of dye granules on the slides, which may cause false positives during the parasite count.
Stage V is the critical gametocyte stage (mature gametocyte) and the one that should be counted. It has an elongated shape with hemozoin pigment granules in the center.
All materials, including a microscope, should be kept ready so that exflagellation counting can start exactly after 10 min and end approximately after 15 min. This process is highly time-sensitive.
The temperature of the incubator for the transformation of gametocytes into ookinetes is very important and should be 24 ± 2°C. The culture should be under very slow shaking conditions (~50 revolutions per min).
It is better to resuspend the pellet with human serum instead of PBS for better adhesion of ookinetes to the glass slides. This prevents the detachment of ookinetes during the washing steps.
Morphologically, P. falciparum ookinetes may resemble stage V gametocytes. To familiarize with differences, consider comparing a gametocyte-containing slide side-by-side with an ookinete-containing slide.
We found that the immersion oil causes gradual fading of Giemsa-stained slides. For this reason it is better to take pictures, thus providing a permanent record.
References
- 1.Ghosh A, et al. The journey of malaria parasite in the mosquito: hopes for the new century. Parasitol Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 2.Janse CJ, et al. Rapid repeated DNA replication during microgametogenesis and DNA synthesis in young zygotes of Plasmodium berghei. Trans R Soc Trop Med Hyg. 1986;80:154–157. doi: 10.1016/0035-9203(86)90219-1. [DOI] [PubMed] [Google Scholar]
- 3.Carter EH, et al. The in vitro cultivation of P. falciparum ookinetes, and their enrichment on Nycodenz density gradients. Parasitology. 1987;95:25–30. doi: 10.1017/s0031182000057516. [DOI] [PubMed] [Google Scholar]
- 4.Sinden RE, et al. The development of Plasmodium ookinetes in vitro : an ultrastructural study including a description of meiotic division. Parasitology. 1985;91:227–244. doi: 10.1017/s0031182000057334. [DOI] [PubMed] [Google Scholar]
- 5.Dinglasan RR, et al. Plasmodium falciparum ookinetes require mosquito midgut chondroitin sulfate proteoglycans for cell invasion. Proc Natl Acad Sci USA. 2007;104:15882–15887. doi: 10.1073/pnas.0706340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh AK, et al. An improved method for the in vitro differentiation of Plasmodium falciparum gametocytes into ookinetes. Malar J. 2010;9:194–200. doi: 10.1186/1475-2875-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bounkeua V, et al. In vitro generation of Plasmodium falciparum ookinetes. Am J Trop Med Hyg. 2010;83:1187–1194. doi: 10.4269/ajtmh.2010.10-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClean CM, et al. Optimized in vitro production of Plasmodium vivax ookinetes. Am J Trop Med Hyg. 2010;83:1183–1186. doi: 10.4269/ajtmh.2010.10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:674–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 10.Carter R, Miller LH. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum continuous culture. Bull World Health Organ. 1979;57:37–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Billker O, et al. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology. 1997;115:1–7. doi: 10.1017/s0031182097008895. [DOI] [PubMed] [Google Scholar]
- 12.Billker O, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]


