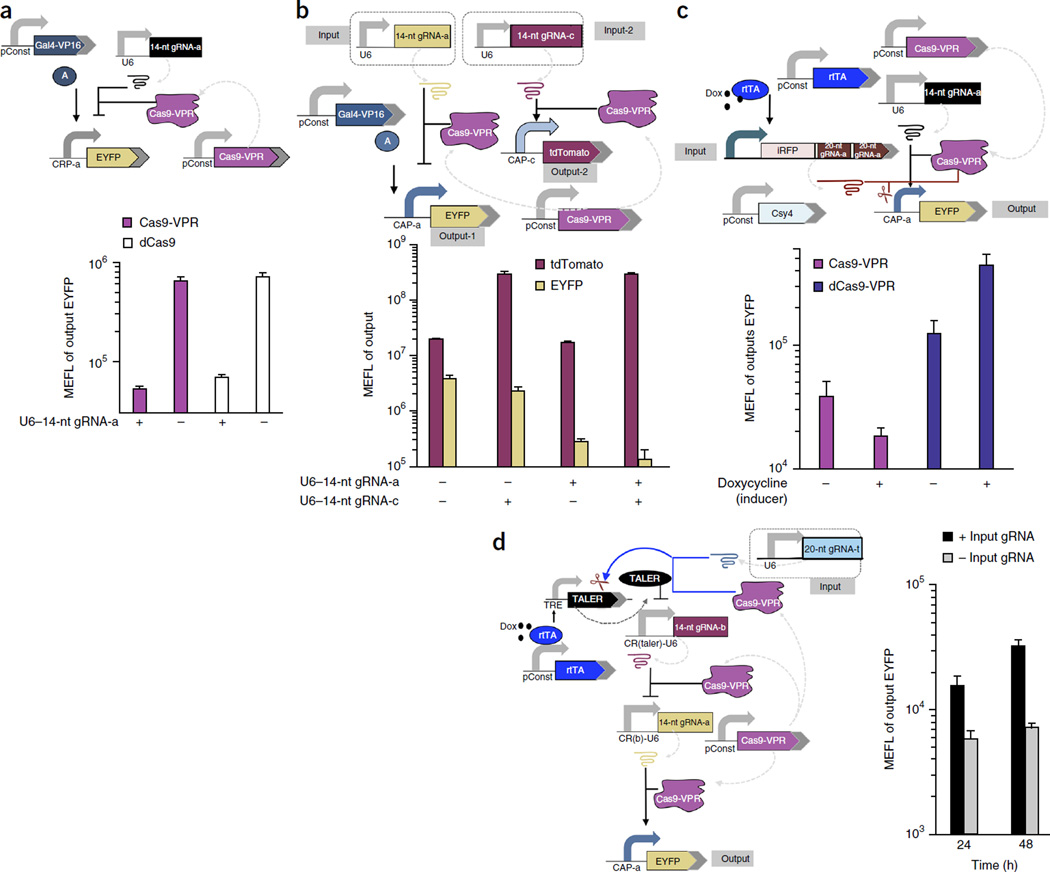
Figure 2.
Design and experimental analysis of human cells with multifunctional CRISPR devices and circuits. (a) Top, schematic of a repression device using Cas9-VPR and 14-nt gRNA. Bottom, the geometric mean and s.d. of EYFP expression for cells expressing >107 molecules of equivalent fluorescein (MEFL) of transfection marker enhanced blue fluorescent protein (EBFP). n = 3 independent technical replicates from three experiments (n = 2 for Cas9-VPR + gRNA). (b) Top, schematic of parallel Cas9-VPR and14-nt gRNA–based transcriptional repression and activation devices in a single cell. Bottom, the geometric mean and s.d. of EYFP and tdTomato expression for cells expressing >107 MEFL of transfection marker EBFP. n = 4 independent technical replicates combined from three experiments. (c) Top, schematic of a genetic kill switch designed to incorporate Cas9-VPR DNA cleavage and transcriptional activation functions. Bottom, the geometric mean and s.d. of EYFP expression for cells expressing >3 × 107 MEFL of transfection marker EBFP. n = 3 independent technical replicates from three experiments (n = 2 for Cas9-VPR without doxycycline). rtTA, reverse tetracycline-controlled transactivator; iRFP, near-infrared fluorescent protein. (d) Left, genetic kill-switch circuit incorporating all three functions of Cas9-VPR: DNA cleavage, transcriptional activation and repression. Right, the geometric mean and s.d. of EYFP expression for cells expressing >107 MEFL of transfection marker mKate. n = 4 independent technical replicates from three experiments (n = 2 in 48-h groups). pConst, constitutively active promoter (e.g., CAG or hEF1-a).