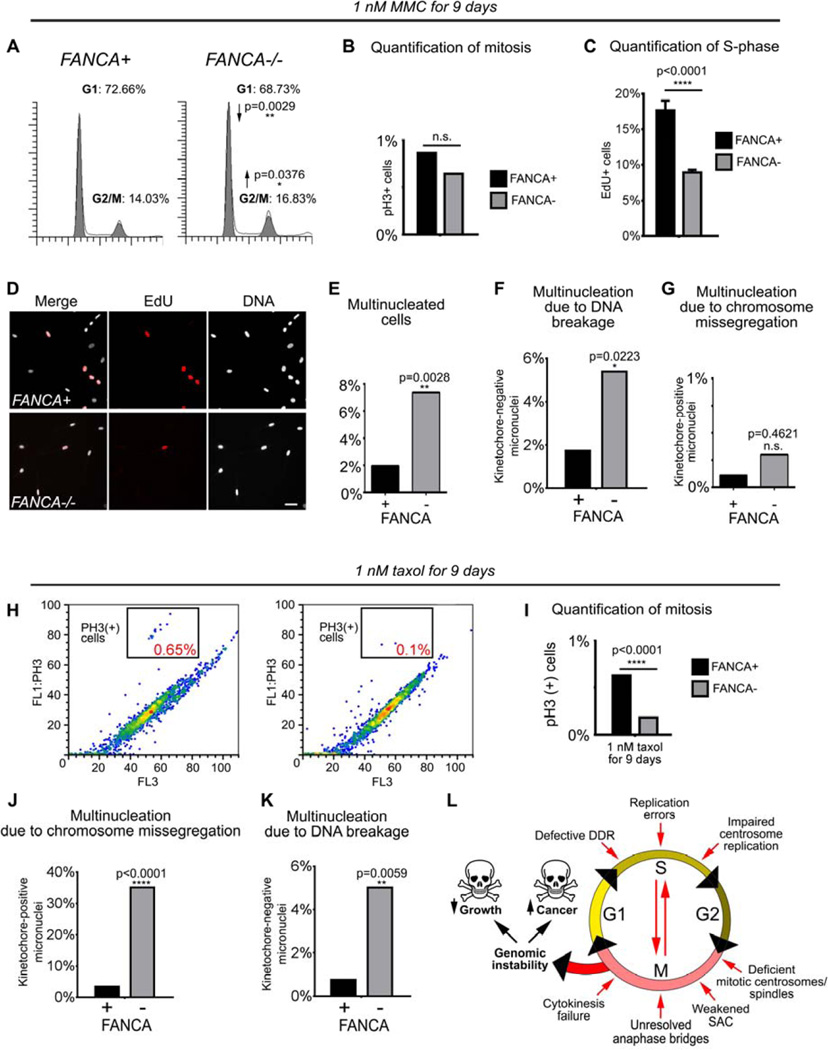
Figure 7. FANCA−/− cells exposed to genotoxic stressors develop genomic instability through a combination of interphase and mitotic checkpoint abnormalities.
(A) Prolonged activation of the G2/M checkpoint in FANCA−/− cells grown in low-dose MMC for 9 days. (B) No difference in mitotic cell fraction between MMC-treated FANCA−/− and FANCA+ cells indicates that the increased FANCA−/− G2/M fraction shown in (A) reflects G2 arrest prior to mitotic entry. (C, D) DNA replication arrest in FANCA−/− cells exposed to 1 nM MMC is rescued by FANCA gene correction. S-phase cells were labeled red by EdU incorporation. (E, F, G) Increased multinucleation due to DNA breakage, but not chromosome missegregation, in FANCA−/− cells grown in low-dose MMC. (H, I) Flow cytometry shows decreased fraction of mitotic cells in FANCA−/− cells exposed to sublethal dose of taxol. (J, K) Treatment with taxol increases chromosome segregation errors and chromosome breakage in FANCA−/− cells. (L) Compound interphase and mitotic origins of genomic instability in FA-deficient cells (see text for discussion). Exponential accumulation of DNA damage may result in activation of cell cycle arrest/apoptosis (bone marrow failure) or malignant transformation (leukemia and solid tumors). All flow cytometry data represent pooled 3 replicates for each cell line and condition compared with two-tailed t-test. EdU incorporation counts were compared via two-way ANOVA with Sidak’s multiple comparisons test.