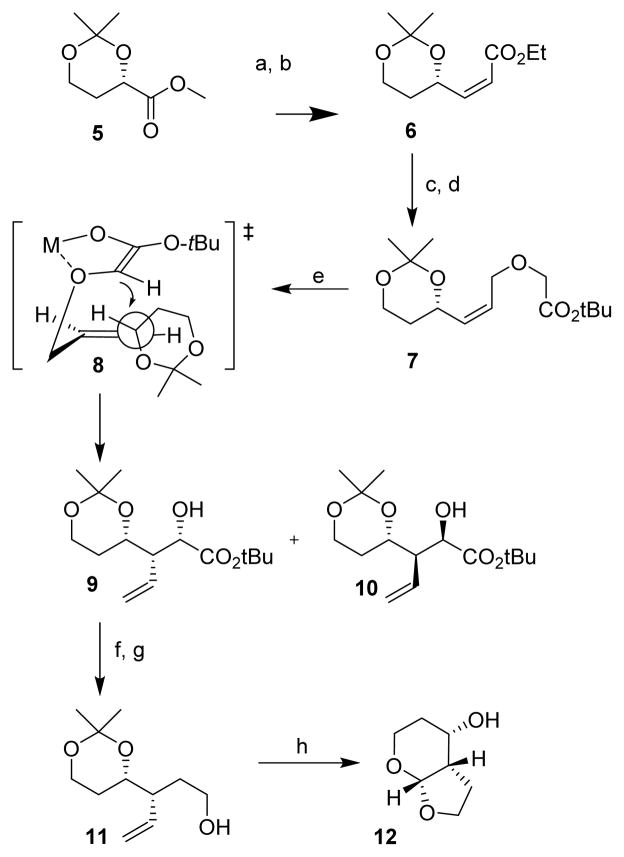
Scheme 1.
Reagents and conditions: (a) Dibal-H, CH2Cl2, −78 °C, 1 h; (b) MeOH, Ph3P=CHCO2Et, 0 °C, 70%; (c) Dibal-H, Ch2Cl2, −78 °C; (d) t-butyl bromoacetate, CsOH·H2O, TBAI, CH3CN, 12 h, 95% 2 steps; (e) LiHMDS, THF, −65 °C −20 °C, 2 h, 77% overall, major/minor (8.5:1); (f) MsCl, Et3N, THF, 0 °C, 1 h; (g) LAH, THF, 23 °C, 24 h, 70% 2 steps; (h) O3, DMS, −78 °C, then p-TsOH, 23 °C, 18 h, 70%.