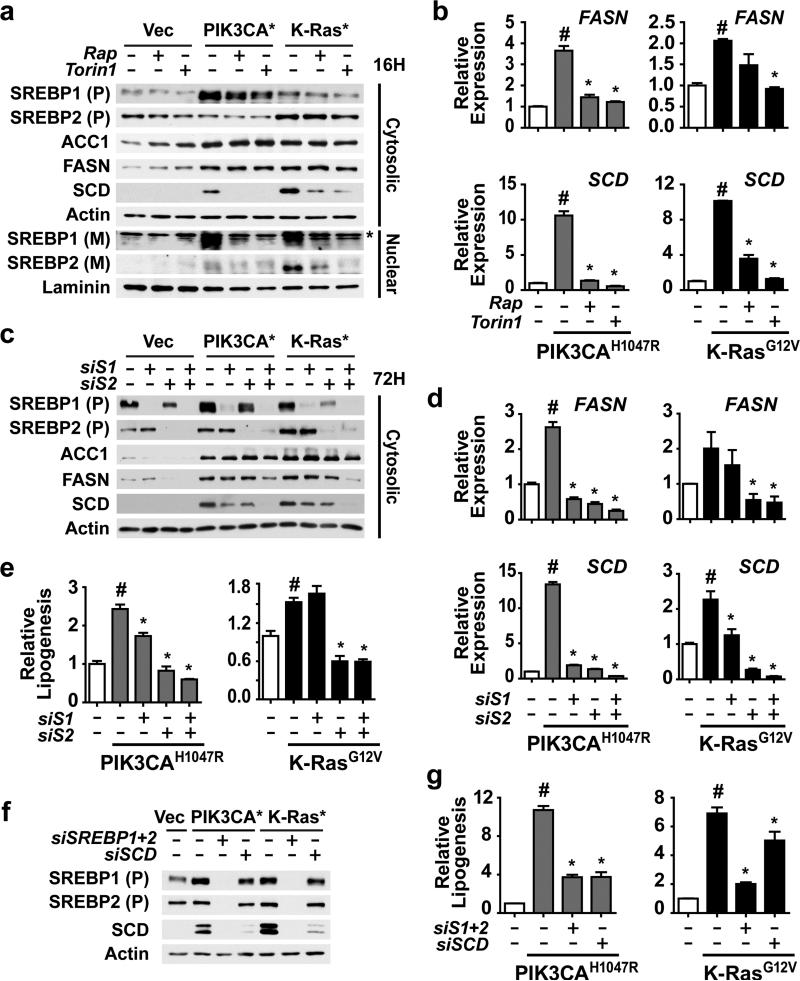
Figure 2.
Activation of SREBP1 and SREBP2 by mTORC1 is required for PI3K- and K-Ras-induced de novo lipogenesis. (a) Regulation of SREBP isoforms by oncogenes and mTORC1. MCF10a cells stably expressing empty vector, PIK3CAH1047R,or K-RasG12V were serum starved for 16 h in the presence of vehicle, rapamycin (20 nM), or Torin1 (250 nM). Cytosolic and nuclear fractions were collected to detect the cytosolic precursor (P) and the nuclear mature (M) forms of SREBP1 and 2. * denotes a cross-reacting band. (b) Oncogene and mTORC1-dependent induction of FASN and SCD expression. RNA was isolated from cells treated as in a for analysis by qRT-PCR. Representative data are shown as mean ± s.e.m. relative to vector-expressing cells (white bar), n=2. (c-e) Effects of SREBP1 and SREBP2 knockdown on SREBP targets and de novo lipogenesis. The cells in a were transfected with siRNAs targeting SREBP1, SREBP2, or both. Cells were lysed 72 h post-transfection following 20 h serum starvation for immunoblotting of the cytosolic fraction (c) or RNA extraction for qRT-PCR analysis (d). Representative data are shown as mean ± s.e.m. relative to vector-expressing cells (white bar), n=3. (e) Incorporation of 1-[14C]-acetate into the lipid fraction was measured in these cells, with data shown as mean ± s.e.m. relative to vector-expressing cells (white bar), n=2. (f,g) Effects of SREBP and SCD knockdown on lipogenesis. The cells in a were transfected with siRNAs targeting SREBP1 and SREBP2, or SCD. Cells were lysed as in c for immunoblotting (f). Incorporation of 1-[14C]-acetate into the lipid fraction was measured in these cells (g). Data are shown as mean ± s.e.m. relative to vector-expressing cells (white bar), n=2. (b,d,e) # P-value < 0.05 compared to vector-expressing cells; * P-value < 0.05 compared to vehicle-treated cells expressing the same oncogene.