Figure 7.
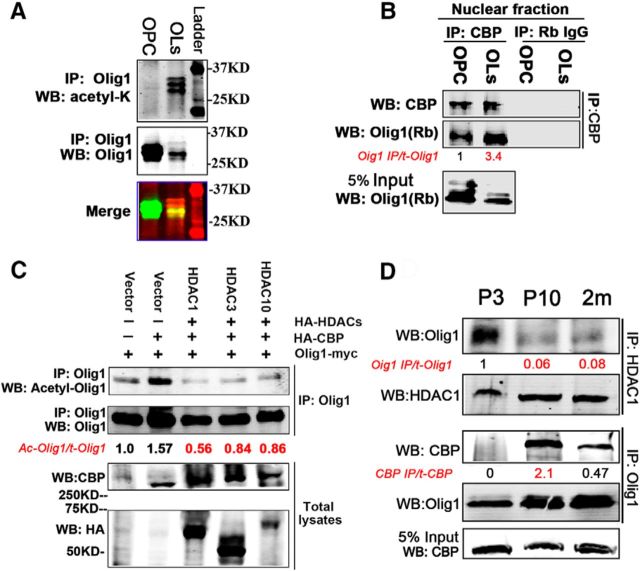
Olig1 acetylation is regulated by preferential interaction with CBP and HDAC1. A, Olig1 was acetylated in cultured rat oligodendrocytes. Cultured rat OPCs or oligodendrocyte cell lysates were immunoprecipitated with an Olig1 antibody and detected with an acetyl-lysine antibody (top) or Olig1 antibody (middle). Merged Western bands are shown in the bottom panel. B, Olig1 interacted with CBP in nuclei of cultured rat oligodendrocytes and this interaction increased as oligodendrocytes differentiated. Nuclear lysates from rat OPCs or differentiated oligodendrocytes were immunoprecipitated with CBP antibody and detected with Olig1 antibody. Quantification of the ratio of immunoprecipitated Olig1 to total Olig1 indicated below in red, normalized to that from OPCs. C, HDAC1, HDAC3, and HDAC10 were potential HDACs responsible for Olig1 acetyl-K150 deacetylation in vivo. The indicated constructs were cotransfected into HEK293T cells. Six hours after transfection, cells were treated with 500 nm TSA overnight and fresh medium was then added for another 10 h. Cell homogenates were immunoprecipitated with Olig1 antibody and detected with acetyl-Olig1-K150 antibody. Quantification of the ratio of acetylated Olig1 to total Olig1 is indicated below in red, normalized to that of empty vector. D, Olig1 preferentially interacted with HDAC1 or CBP in developing mouse brain. Endogenous HDAC1 was immunoprecipitated from subcortical white matter homogenates from different postnatal developmental ages and bound Olig1 was detected with Olig1 antibody as indicated (top row). Endogenous Olig1 was immunoprecipitated from the same samples and bound CBP was detected with CBP antibody (middle row). Quantification of the ratio of immunoprecipitated Olig1 to total Olig1 (normalized to that from P3 tissue) and immunoprecipitated CBP to total CBP indicated below in red, normalized to that from OPCs. Note the high association of Olig1 and HDAC1 in P3 tissue and high association of Olig1 and CBP in P10 tissue.