Abstract
Brainstem catecholamine neurons modulate sensory information and participate in control of cardiorespiratory function. These neurons have multiple projections, including to the paraventricular nucleus (PVN), which contributes to cardiorespiratory and neuroendocrine responses to hypoxia. We have shown that PVN-projecting catecholaminergic neurons are activated by hypoxia, but the function of these neurons is not known. To test the hypothesis that PVN-projecting catecholamine neurons participate in responses to respiratory challenges, we injected IgG saporin (control; n = 6) or anti-dopamine β-hydroxylase saporin (DSAP; n = 6) into the PVN to retrogradely lesion catecholamine neurons projecting to the PVN. After 2 wk, respiratory measurements (plethysmography) were made in awake rats during normoxia, increasing intensities of hypoxia (12, 10, and 8% O2) and hypercapnia (5% CO2-95% O2). DSAP decreased the number of tyrosine hydroxylase-immunoreactive terminals in PVN and cells counted in ventrolateral medulla (VLM; −37%) and nucleus tractus solitarii (nTS; −36%). DSAP produced a small but significant decrease in respiratory rate at baseline (during normoxia) and at all intensities of hypoxia. Tidal volume and minute ventilation (VE) index also were impaired at higher hypoxic intensities (10-8% O2; e.g., VE at 8% O2: IgG = 181 ± 22, DSAP = 91 ± 4 arbitrary units). Depressed ventilation in DSAP rats was associated with significantly lower arterial O2 saturation at all hypoxic intensities. PVN DSAP also reduced ventilatory responses to 5% CO2 (VE: IgG = 176 ± 21 and DSAP = 84 ± 5 arbitrary units). Data indicate that catecholamine neurons projecting to the PVN are important for peripheral and central chemoreflex respiratory responses and for maintenance of arterial oxygen levels during hypoxic stimuli.
Keywords: chemoreflex, blood pressure, ventilation, anti-dopamine β-hydroxylase saporin, brainstem
peripheral chemoreflex activation by systemic hypoxia produces highly integrated respiratory, autonomic, behavioral, and endocrine responses (15, 17). Together these responses are critical for homeostasis, serving to restore and maintain tissue oxygenation. The central nervous system pathways involved in responses to acute hypoxia are complex. Peripheral chemoreceptor afferent nerves from the carotid bodies project onto neurons located in the nucleus tractus solitarii (nTS) (40, 51), where chemoreceptor afferent input is modulated and integrated. The nTS sends projections to the rostral ventrolateral medulla (RVLM) (28, 30), and this projection is considered the primary pathway mediating cardiorespiratory chemoreflex responses.
The hypothalamic paraventricular nucleus (PVN) is also important in the integrated response to chemoreflex activation. PVN neurons are activated by peripheral and central chemoreflex stimulation, including hypoxia and hypercapnia (3, 11, 22). Furthermore, the PVN is required for full expression of the chemoreflex pressor, sympathoexcitatory and respiratory responses (42, 47). However, the pathways by which chemoreflex afferent information is relayed to the PVN have not been thoroughly defined. The PVN receives catecholaminergic input from adrenergic/noradrenergic regions in the nTS (A2 and C2 cell groups), ventrolateral medulla (VLM; A1 and C1 groups), and locus coeruleus (A6) (9, 52). These catecholaminergic populations respond and contribute to responses to a variety of cardiorespiratory stimuli (7, 22, 24, 48). For example, A1 noradrenergic neurons in the caudal VLM (CVLM) are activated by hypoxia (26, 55), and CVLM lesion attenuates vasopressin and corticotropin-releasing hormone (CRH) responses to hypoxia (55). Furthermore, PVN adrenergic blockade blunts cardiovascular and vasopressin responses to acute chemoreflex stimulation in spinally transected rats (32).
Recent studies have shown that acute hypoxia activates PVN-projecting neurons in both the nTS and VLM. Importantly, a large proportion of these projection neurons is catecholaminergic (24, 26). However, the extent to which catecholaminergic input to the PVN plays a functional role in cardiorespiratory responses to acute hypoxia is unknown. This study tested the hypothesis that catecholaminergic inputs to the PVN participate in cardiorespiratory responses to hypoxia or hypercapnia. To test this hypothesis, we retrogradely lesioned PVN-projecting catecholaminergic neurons by injecting anti-dopamine β-hydroxylase conjugated to the ribosomal inactivating protein saporin (DSAP) into the PVN of rats and measured ventilation, arterial blood pressure, and heart rate during normoxic, hypoxic, and hypercapnic conditions. Overall, the present study demonstrates the importance of catecholaminergic inputs to the PVN in mediating chemoreflex-induced responses. This study has been presented previously in abstract form (25).
METHODS
Animals
Adult male Sprague-Dawley rats were obtained from Harlan Laboratories. Animals were housed individually in a temperature- and humidity-controlled vivarium and maintained on a 12:12-h light-dark cycle with food and water available ad libitum. All experimental procedures were approved by the University of Missouri Institutional Animal Care and Use Committee and conform to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (250–350 g; n = 12) were assigned to two groups: lesion with PVN microinjection of DSAP (n = 6) or control with IgG-saporin (n = 6).
Surgical Procedures
Two weeks before the hypoxia experiments, rats were anesthetized with isoflurane (5% for induction and 2–2.5% for maintenance, AErane; Baxter, Deerfield, IL). The following procedures were performed using aseptic technique.
Telemetry device placement for cardiovascular measurements.
To assess continuous mean arterial blood pressure (MAP) and heart rate (HR) changes in response to acute hypoxia, all rats were instrumented with a telemetry device (TA11PA-C40; Data Sciences International) in the abdominal aorta, as described previously (24). Briefly, following anesthesia, a midline incision was made, the abdominal aorta was visualized, and the catheter probe of the telemetry device was inserted. The site was sealed with a cellulose patch and tissue adhesive. The transmitter was secured to the abdominal muscle using a nonabsorbable suture and the skin incision closed.
Immunotoxin lesions.
During the same surgery, DSAP (42 ng in 200 nl of phosphate buffer, pH 7.4, n = 6; Advanced Targeting Systems) or a control IgG (Advanced Targeting Systems, 42 ng in 200 nl in phosphate buffer, pH 7.4, n = 6) was microinjected bilaterally into the PVN. Importantly, DSAP is retrogradely transported (46, 49, 60) and, therefore, selectively eliminates catecholaminergic neurons that project to the PVN. Injections were made through a stereotaxically positioned glass micropipette [coordinates: 1.8–2.0 mm caudal to Bregma, ±0.5 mm lateral from the midline, and 7.6–7.8 mm ventral to the dura (24, 35, 38)]. The concentration and volume of DSAP and IgG injected were determined from previous experiments using similar protocols (4, 12). Rats were treated postoperatively with fluids (3 ml sc, 0.9% saline), enroflaxin (2.5 mg/kg im; Bayer, Shawnee Mission, KS), and buprenorphine (0.03 mg/kg sc; Reckitt Benckiser Pharmaceuticals, Richmond, VA) for hydration, prevention of infection, and pain management, respectively. Upon recovery from anesthesia, the animals were returned to their cages. At least 2 wk were allowed for degeneration of the targeted neurons and for recovery from surgery. During this period, daily clinical examination, body weight measurements, and the telemetry signal were evaluated.
Plethysmography for Ventilatory Assessment
In conscious, unrestrained rats, ventilation was assessed by whole body plethysmography (24, 27). One DSAP- and one IgG-treated rat were placed in adjoining whole body plethysmography chambers and experiments run simultaneously using the same gas sources. Chambers (Data Sciences International) included inlet and outlet ports to allow airflow; the animal chamber and a reference chamber were connected to a differential pressure transducer (Validyne MP45; Validyne Engineering). The recorded pressure signal is proportional to volume changes and provided a measurement of tidal volume by integrating the area under the inspiratory pressure curve (tidal volume index). Ventilatory measurements were recorded using a data acquisition system (PowerLab; ADInstruments, Colorado Springs, CO) and included respiratory rate (breaths/min), tidal volume index, and minute ventilation index (respiratory rate × tidal volume index). We also examined the number and amplitude of augmented breaths. Tidal volume index, minute ventilation index, and augmented breath amplitude were normalized to body weight. Oxygen saturation was measured using a collar pulse oximeter (MouseOx; Starr Life Sciences).
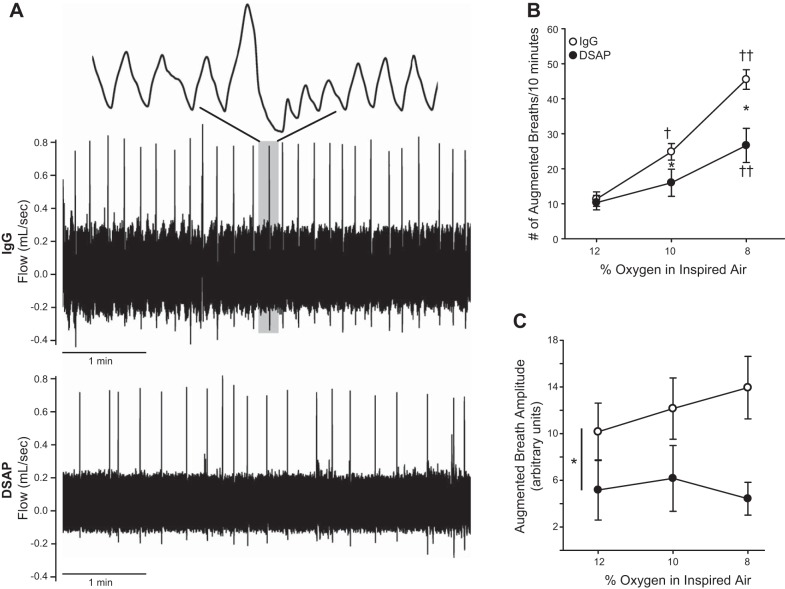
Baseline ventilation was assessed in conscious animals before surgical procedures and on the day of the experiment examining chemoreflex function. Because telemetry devices were implanted during the surgery, cardiovascular parameters were measured on the day of the experiment only. Animals were acclimatized to the chamber environment for 3 days before the actual experiments. On the day of the experiment, animals were allowed to acclimate to the plethysmograph while breathing room air. Ventilation, oxygen saturation, arterial blood pressure, and HR were monitored continuously during exposure to 20 min of normoxia (21% O2) and hypoxia (12, 10, and 8% O2) to assess peripheral chemoreflex function. To evaluate central chemoreflex (CO2) responses, rats were exposed (20 min) to hyperoxia (100% O2) to minimize peripheral chemoreceptor input, followed by hypercapnia (95% O2-5% CO2). The order of gas exposure was varied to produce a balanced design, except that 100% O2 was always followed by hypercapnia to isolate central chemoreflex responses. For experiments examining responses to hypoxia, mixtures of gases were regulated by a gas blender that provided precise control of oxygen concentrations (Hypoxydial; Starr Life Sciences), which were confirmed by an O2 analyzer; hypercapnia experiments utilized compressed gas tanks. The ventilatory response during these exposures was quantified by analyzing an average of approximately 20 consecutive breaths, excluding sniffs, augmented breaths, or movement artifacts. Augmented breaths were determined from their comparatively large inspiratory and expiratory flows, which could easily be discerned from the background of normal breathing, appearing as episodic spikes in the trace (Fig. 4A). Because of their relatively infrequent occurrence in our 20 min of normoxic exposure, frequency of augmented breaths was not analyzed during normoxia and was expressed as the number of augmented breaths per 10 s during hypoxic exposures.
Fig. 4.
Lesion of PVN-projecting catecholaminergic neurons reduced the number of augmented breaths during hypoxia. A: representative flow traces from a control (top) and DSAP-lesioned (bottom) animal while breathing 8% O2. Augmented breaths appear as large amplitude “spikes” in the traces with compressed time scales (5 min). The top trace is an expanded time scale of the region in gray for an augmented breath. There was a decreased prevalence of augmented breaths in the DSAP compared with the IgG-treated animal. B: mean data indicate that with decreasing O2, control animals exhibit a progressive increase in the number of augmented breaths. In DSAP animals, there were significantly fewer augmented breaths at 10 and 8% O2 (2-way repeated-measures ANOVA). C: DSAP decreased the amplitude of augmented breaths (main effect, 2-way repeated-measures ANOVA). *P ≤ 0.05 vs. IgG; †P ≤ 0.05 vs. 12% O2; ††P ≤ 0.05 vs. 12 and 10% O2.
Assessment of DSAP Lesion and Immunohistochemistry
After chemoreflex testing, animals were transcardially perfused with heparinized, oxygenated Dulbecco's modified Eagle's medium (125 ml, pH 7.4; Sigma), followed by 4% paraformaldehyde (500 ml, pH 7.4; Sigma). Brains were removed and postfixed overnight in 4% paraformaldehyde, followed by coronal sectioning (30 μm) on a vibratome (VT 1000S; Leica). To evaluate the efficacy of DSAP lesions, standard immunohistochemical approaches were used to label tyrosine hydroxylase (TH) cells in the brainstem. TH terminals in the PVN were evaluated in a subset of IgG- (n = 4) and DSAP-treated (n = 3) rats. TH, the rate-limiting enzyme in catecholamine synthesis, was used as a marker in classifying neurons as catecholaminergic (28, 45). Immunohistochemistry was performed simultaneously on tissue from IgG- and DSAP-treated rats. Every sixth section was incubated overnight in 1% normal donkey serum (NDS) and 0.3% Triton-0.01 M PBS containing primary antibody against TH (mouse anti-TH, 1:1,000; Millipore). Sections were then rinsed and incubated for 2 h in Cy2-conjugated donkey anti-mouse IgG (1:200; Jackson ImmunoResearch) with 1% NDS in 0.3% Triton-0.01 M PBS, mounted on slides, and coverslipped for microscopic evaluation. Images of histological sections were captured using an Olympus fluorescence microscope (BX51). Identical exposures were used to capture images of tissue from DSAP- and IgG-treated animals. Images were analyzed with ImageJ (version 1.41; National Institutes of Health) using a custom-made plugin (GAIA Group, Novato, CA; http://gaiag.net/index.html). Brightness and contrast only were altered equally in tissue from DSAP and IgG animals. Two individuals blinded to experimental groups counted the number of TH-immunoreactive (TH-IR) cells, and results were averaged. For individual brain regions, cells were counted over a specific range relative to Bregma based on an anatomic brain atlas (43): (nTS, A2 cell group: −14.82 to −13.74 mm; VLM, A1 and C1 cell groups: −15.0 to −12.12 mm). All TH-positive cells with distinct cytosolic labeling, visible processes, and a blank nuclear area were counted in each brain region of interest. PVN terminals were evaluated (ImageJ) using the same demarcated area from IgG- and DSAP-treated tissue. Thresholding was performed based on control tissue. The overall area of immunopositive labeling within the defined region was measured, and the number of putative terminals was determined by particle analysis.
Statistics
Statistical analyses were performed using SigmaPlot (12.3; Systat Software, San Jose, CA). Power analyses indicate adequate power for all group comparisons and all comparisons among hypoxic intensities, except in the case of amplitude of augmented breaths. All data are presented as means ± SE, and statistical significance was set at P ≤ 0.05. Total counts of TH-IR neurons were compared between IgG and DSAP treatment by t-test. Caudal-rostral distributions of TH labeling were analyzed by two-way repeated-measures analysis of variance (ANOVA). A two-way repeated-measures ANOVA was performed to examine differences in cardiorespiratory responses of the two groups (IgG and DSAP injection) to various levels of inspired O2 or CO2. Pearson correlation was used to calculate r2 values, slope, and significance of correlations between ventilatory responses and oxygen saturation. t-tests were used to compare the slopes of respiratory variables plotted against oxygen saturation. When interactions between group and oxygen level occurred, ANOVAs were followed by post hoc analysis using Fisher's least significant difference test.
RESULTS
Lesion of PVN-Projecting Catecholaminergic Neurons Decreases the Number of TH-IR cells in the Brainstem
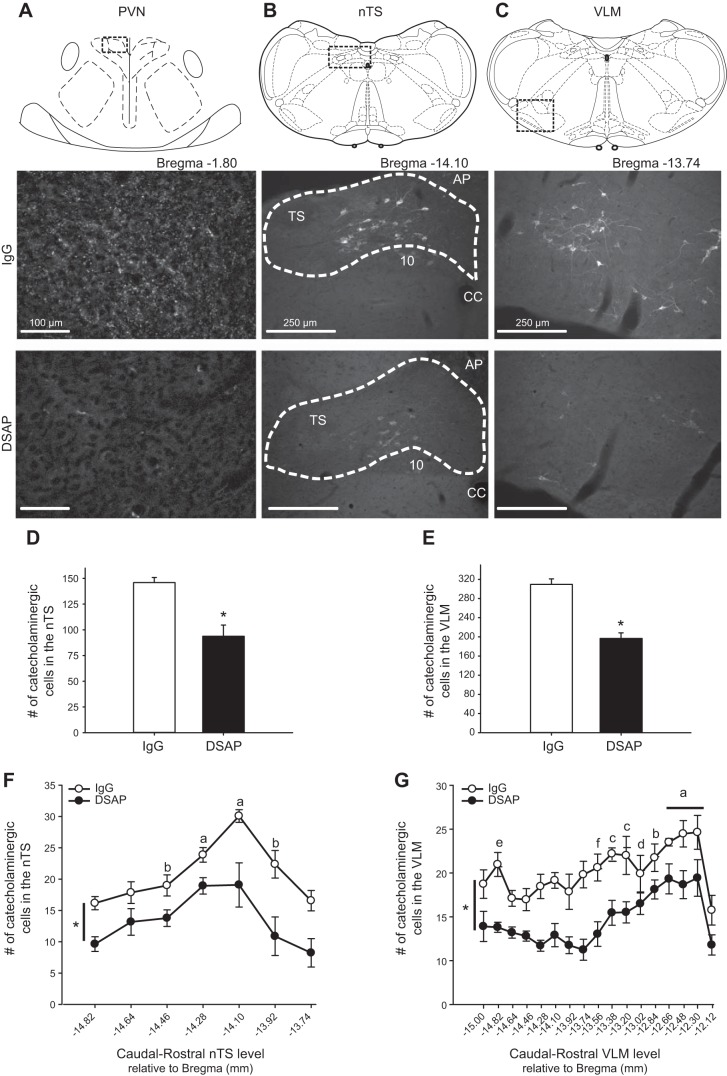
To validate the efficacy of DSAP (14, 49) to lesion PVN-projecting catecholaminergic neurons, we examined TH-IR in the PVN. Figure 1A shows the region of PVN evaluated (top) and punctate TH-IR (bottom) in the PVN of an IgG- compared with a DSAP-treated rat. DSAP reduced overall TH-IR within the PVN by 97% (%area, IgG: 0.634 ± 0.191; DSAP: 0.020 ± 0.006, P ≤ 0.05) and the number of putative terminals by 96% (IgG: 191 ± 60; DSAP: 7 ± 1, P ≤ 0.05). We also evaluated elimination of TH-IR neurons in the brainstem, specifically the regions of the nTS and VLM. Figure 1, B and C, includes schematic representations (top) (43) and corresponding photomicrographs of coronal sections (bottom) showing TH-IR in nTS and VLM of IgG- and DSAP-treated rats, respectively. DSAP injections significantly reduced the number of catecholaminergic (TH-IR) neurons within the nTS and VLM (Fig. 1, D and E). DSAP decreased the number of TH-IR cells by 36% in the nTS and by 37% in the VLM.
Fig. 1.
Anti-dopamine β-hydroxylase conjugated to the ribosomal-inactivating protein saporin (DSAP) injected into the PVN reduces tyrosine hydroxylase-immunoreactive (TH-IR) terminals in the paraventricular nucleus (PVN) and catecholaminergic neurons in both nucleus tractus solitarii (nTS) and ventrolateral medulla (VLM) regions. A: schematic representation (top) and photomicrographs of TH-IR terminals in the boxed region of PVN from an IgG- (middle) and a DSAP-treated rat (bottom). DSAP treatment reduced TH-IR in the PVN. B and C: schematic representations (top) of the areas corresponding to photomicrographs (bottom) containing the nTS (Bregma −14.10 mm; B) and VLM (Bregma −13.74 mm; C). Boxes indicate the regions represented in the images. Photomicrographs show TH-positive cells in representative IgG- and DSAP-treated animals. DSAP lesions reduced TH-IR in both regions. Dashed area represents the region counted. D and E: total no. of TH-positive neurons counted within the nTS (D) and VLM (E) in IgG- (open bars) and DSAP-treated rats (filled bars). F and G: caudal to rostral extent (relative to Bregma) of the nTS (F) and VLM (G) illustrating the loss of catecholaminergic cells. DSAP lesions significantly reduced the number of catecholaminergic neurons within both the nTS and VLM. P ≤ 0.05. *Main effect of treatment (DSAP < IgG controls). There also was a main effect of caudal to rostral distribution in both nTS and VLM. nTS (F): a > b and −14.64, −14.46, and −13.92; b > −13.74 and −14.82. VLM (G): a > b and −14.82, −13.02, −13.20, and −13.38; b > c and −15.00 and −13.56; c > d and −14.1; d > e and −14.46, −14,28, −14.64, and −13.74; e > f and −13.92; f > −12.12. 10, dorsal motor nucleus of the vagus; AP, area postrema; CC, central canal; TS, tractus solitarii.
Analysis of distribution of catecholamine cells indicated a main effect of caudal-rostral level in both nTS and VLM (Fig. 1, F and G). For nTS, most TH-IR cells were at the level of calamus scriptorius and rostral (−14.28 to −14.10 relative to Bregma), whereas in the VLM, the greatest number was located more rostrally. Importantly, there also was a main effect of treatment in both regions, indicating that DSAP injections produced a loss of TH-IR cells through both the nTS and VLM. The reduction due to DSAP in nTS catecholaminergic cells at the level of the area postrema (Bregma −14.10 to −13.74, 46 ± 5%) was consistent with the percentage of PVN-projecting neurons in this region reported previously (21). Within the VLM, the relative decrease in number of TH-IR cells due to DSAP (−43 ± 2%) was significantly greater in sections at and caudal to Bregma −13.2 compared with more rostral sections (−27 ± 2%), consistent with previous work showing that a portion of these rostral VLM catecholaminergic cells do not project to the PVN (19, 54).
Deletion of PVN-Projecting Catecholamine Neurons Alters Baseline Respiratory Rate and Blood Pressure
Prior to surgical procedures, body weight, oxygen saturation, and ventilation during normoxic breathing were not different between rats given IgG control or DSAP (Table 1). Two weeks postinjection, body weight increased in both groups. There was no significant difference between groups in body weight, tidal volume, and minute ventilation. Respiratory rate, tidal volume, and minute ventilation were similar pre- and postinjection of IgG. In contrast, DSAP-injected animals exhibited a small but statistically significant decrease in respiratory rate while breathing room air (21% O2) compared with both preinjection and the control group. In addition, DSAP injections into the PVN resulted in a small but significant increase in baseline MAP compared with IgG controls. Because telemetric devices were implanted during the same surgery as PVN injections, presurgical measurements of MAP and HR were not obtained.
Table 1.
Effects of IgG or DSAP microinejctions into the PVN on baseline cardiorespiratory parameters
| Preinjection |
Postinjection |
|||
|---|---|---|---|---|
| IgG | DSAP | IgG | DSAP | |
| Body weight, g | 241 ± 23 | 230 ± 16 | 302 ± 14† | 279 ± 4† |
| Oxygen saturation, % | 95.7 ± 0.2 | 95.9 ± 0.4 | 95.9 ± 0.6 | 95.7 ± 0.4 |
| Respiratory rate | 95 ± 5 | 94 ± 3 | 88 ± 2 | 76 ± 1*† |
| Tidal volume index | 1.37 ± 0.42 | 1.29 ± 0.03 | 1.32 ± 0.1 | 1.22 ± 0.05 |
| Minute ventilation index | 123 ± 30 | 121 ± 5 | 116 ± 8 | 93 ± 4 |
| Mean arterial pressure, mmHg | 112 ± 1 | 122 ± 1.5* | ||
| Heart rate, beats/min | 353 ± 7 | 367 ± 15 | ||
All values are means ± SE.
DSAP, anti-dopamine β-hydroxylase saporin; PVN, paraventricular nucleus.
Preinjection values were not significantly different between groups (n = 6 each). After DSAP injection, baseline respiratory rate was decreased and mean arterial pressure increased.
P ≤ 0.05 vs. IgG;
P ≤ 0.05 vs. preinjection within a group. Mean arterial pressure and heart rate were measured using telemetric devices implanted at the time of injection. Therefore, no preinjection measurements were made.
PVN-Projecting Catecholamine Neurons Contribute to Cardiorespiratory Responses to Hypoxia
To evaluate the role of PVN-projecting catecholaminergic neurons in responses to hypoxia, we compared ventilatory responses to hypoxia in IgG control animals with those of DSAP-lesioned animals. Figure 2A contains representative recordings of plethysmography-acquired respiratory responses from individual rats with PVN injections of IgG (Fig. 2A, top) or DSAP (Fig. 2A, bottom) to normoxia (21% O2) or activation of the peripheral chemoreflex with three intensities of acute hypoxia. Respiratory variables increased within the first minute of hypoxic challenge and remained elevated throughout the hypoxia exposures. The progressive increase in respiratory rate and tidal volume due to increased hypoxic intensity was less in the DSAP- compared with the IgG-treated rat. Group data for respiratory variables are shown in Fig. 2, B–E. In both groups, increasing hypoxia progressively decreased arterial oxygen saturation and increased respiratory rate, tidal volume index, and minute ventilation index. In the DSAP group, however, oxygen saturation was less compared with IgG control animals at each intensity of hypoxia. Despite lower oxygen saturation and presumably partial pressures of oxygen in DSAP-treated animals, tidal volume and minute ventilation during exposure to 12% O2 were similar to IgG-injected rats. Furthermore, ventilatory responses during higher intensities of hypoxia were blunted in DSAP rats.
Fig. 2.
Lesions of PVN-projecting catecholaminergic neurons blunted ventilatory responses to hypoxia. A: plethysmography traces showing changes in breathing in individual awake IgG control (top) and DSAP-injected rats (bottom) during normoxia and 3 intensities of hypoxia (10 s each). B–G: group data (n = 6 each) showing effect of graded hypoxia on oxygen saturation (B), respiratory rate (C), tidal volume index (D), minute ventilation index (E), mean arterial pressure (F), and heart rate (G) in IgG (○) vs. DSAP-treated animals (●) animals. Two-way repeated-measures ANOVA indicated significant interactions of hypoxia intensity and group in all respiratory variables and heart rate and a main effect of hypoxia for mean arterial pressure. DSAP injections significantly decreased respiratory rate during normoxia and blunted ventilatory responses to increasing intensities of hypoxia despite greater decreases in oxygen saturation. *P ≤ 0.05 vs. IgG; †P ≤ 0.05 vs. 21% O2; ††P ≤ 0.05 vs. 21 and 12% O2; †††P ≤ 0.05 vs. 21, 12, and 10% O2 within a group.
In both the IgG- and DSAP-treated groups, systemic hypoxia evoked a decrease in arterial blood pressure, although MAP in DSAP-treated animals was higher during all intensities of hypoxia (Fig. 2F). In IgG control animals, HR increased during hypoxia, reaching statistical significance at 8% O2. In contrast, HR remained unaltered in rats treated with DSAP (Fig. 2G).
Because oxygen saturation during hypoxia was different between groups, we also evaluated the respiratory response relative to measured oxygen saturation (Fig. 3). In both groups, a decline in oxygen saturation was associated with a progressive increase in respiratory rate, tidal volume, and minute ventilation. However, the slope of these relationships was significantly reduced in DSAP-treated rats compared with controls (Table 2), indicating an attenuated response to a given arterial oxygen saturation. Overall, these results indicate that bilateral lesion of PVN-projecting catecholaminergic neurons markedly attenuated cardiorespiratory responses to hypoxia.
Fig. 3.
Correlation of ventilatory responses and oxygen saturation in IgG vs. DSAP-injected animals. Respiratory rate (A), tidal volume index (B), and minute ventilation index (C) responses relative to oxygen saturation in IgG- (○) vs. DSAP-injected animals (●). The slope of each correlation (Table 2) was significantly less in DSAP-injected animals compared with control. *P ≤ 0.05 vs. IgG.
Table 2.
Slope of each parameter relative to oxygen saturation and r2 values for the correlation between ventilatory responses and oxygen saturation in IgG- vs. DSAP-injected animals
| IgG | DSAP | |
|---|---|---|
| Slope | ||
| Respiratory rate | −1.92 ± 0.37 | −0.88 ± 0.09* |
| Tidal volume | −0.03 ± 0.01 | −0.009 ± 0.0006* |
| Minute ventilation | −4.96 ± 1.03 | −1.38 ± 0.06* |
| r2 | ||
| Respiratory rate | 0.89 ± 0.03 | 0.90 ± 0.03 |
| Tidal volume | 0.83 ± 0.02 | 0.79 ± 0.08 |
| Minute ventilation | 0.89 ± 0.02 | 0.85 ± 0.04 |
All values are means ± SE. Slope and r2 values were calculated for the relationship of ventilatory responses and oxygen saturation shown in Fig. 3. The slope of each correlation was significantly less in DSAP-injected animals compared with controls.
P < 0.05 vs. IgG.
A common feature of chemoreflex responses is the occurrence of augmented breaths taken during hypoxia. Figure 4A contains raw flow traces obtained from individual rats injected with IgG (Fig. 4A, top) or DSAP (Fig. 4A, bottom) during an 8% O2 challenge and indicates that augmented breaths were more prevalent in the IgG-treated compared with the DSAP-treated rat. The mean frequency of augmented breaths is displayed in Fig. 4B. Augmented breaths were rarely observed during normoxia in our 20-min experimental period, and data were not analyzed. Both IgG- and DSAP-treated groups displayed augmented breaths in response to 12% O2. In IgG-injected animals, the frequency increased progressively at 10 and 8% O2. However, in DSAP-injected animals, frequency was not significantly increased further until exposure to 8% O2. Furthermore, augmented breath frequency was significantly less in DSAP compared with control IgG-treated animals while breathing 10 and 8% O2. There was no significant effect of hypoxia intensity on the amplitude of augmented breaths (Fig. 4C), although it should be noted that variability was high and the power for detecting hypoxia-related differences was low. Nevertheless, amplitude of augmented breaths was significantly less in DSAP-treated animals. These results indicate that augmented breaths during hypoxia are dependent in part on catecholaminergic input to the PVN.
PVN-Projecting Catecholamine Neurons Contribute to Cardiorespiratory Responses to Hypercapnia
To evaluate the importance of PVN-projecting neurons in central (CO2) chemoreceptor function, we first used 100% O2 to minimize peripheral chemoreceptor input, followed by exposure to 5% CO2-95% O2 to activate predominantly central chemoreceptors (16, 29). Figure 5A shows respiratory flow traces during hyperoxia (100% O2; Fig. 5A, left), followed by hypercapnia (5% CO2-95% O2; Fig. 5A, right) in individual rats with control (IgG) or DSAP injections in the PVN. Mean data (Fig. 5, B–D) show that ventilation during inhalation of 100% O2 was similar in IgG- and DSAP-treated rats. Hypercapnia increased respiratory rate, tidal volume, and minute ventilation relative to breathing 100% O2 in both groups. However, DSAP-injected animals were significantly less responsive to hypercapnia compared with control animals with respect to all ventilatory parameters.
Fig. 5.
The central CO2 chemoreflex response is blunted after lesion of catecholaminergic cells projecting to the PVN. Plethysmography traces showing breathing during 100% O2 (left) and 5% CO2-95% O2 (right) in individual IgG control (top) vs. DSAP animals (bottom). Group data (n = 6) showing effect of 100% O2 compared with 5% CO2-95% O2 on respiratory rate (B), tidal volume index (C), and minute ventilation index (D) in control (open bars) vs. DSAP animals (black bars). There was a significant increase in all ventilatory parameters between 100% O2 and 5% CO2-95% O2 in both groups. However, the response was blunted in DSAP- vs. IgG-injected animals. *P ≤ 0.05, DSAP vs. IgG; †P ≤ 0.05 vs. 100% within a group (IgG or DSAP).
DISCUSSION
This study provides new findings regarding cardiorespiratory control by PVN-projecting catecholaminergic neurons. Depletion of neurons in primary catecholaminergic brainstem regions depressed respiratory rate slightly and increased arterial pressure during normoxia, suggesting that catecholaminergic inputs to PVN may contribute to baseline ventilation and cardiovascular control. Importantly, lesioning these projections reduced ventilatory responses to hypoxia and diminished the ability to maintain O2 saturation. Responses to central chemoreflex activation by hypercapnia were also impaired. These findings indicate that PVN-projecting catecholaminergic neurons are integral to cardiorespiratory adjustments to both peripheral and central chemoreflex challenges and may play a role in baseline cardiorespiratory control.
PVN injections of DSAP were used to selectively lesion catecholamine cells projecting to PVN. Similar to previous studies (4, 49), catecholamine terminals in PVN were decreased and cell numbers were significantly reduced in nTS and VLM, regions that provide the majority of catecholaminergic inputs to PVN (9, 44, 52). In studies using PVN injections of DSAP (4, 12–14, 49, 50), the extent of catecholamine depletion in specific brain regions has been variable (∼20–80%). Depletion in the current experiments was within this range and consistent with our previous work (24, 26) examining nTS and VLM catecholaminergic neurons projecting to PVN. The percentage of catecholaminergic neurons lesioned and depletion of PVN terminals in the present study suggest that we were able to selectively lesion catecholaminergic neurons with PVN connections, and plethysmography data indicate that these neurons are integral to cardiorespiratory regulation.
Studies in mutant mice with impaired development of catecholaminergic neurons (59) and in rats with widespread loss of catecholaminergic cells due to DSAP administration into the fourth cerebral ventricle (33) indicate that these neurons provide a tonic stimulus to intrinsic breathing frequency. Current experiments confirm and extend these findings, indicating that in conscious rats, catecholaminergic projections specifically to PVN play a significant role in baseline respiratory rate. Our data contrast with a recent study (2) suggesting that reducing overall nTS catecholamine synthesis using shRNA had no effect on basal respiratory frequency. Several possibilities may account for this discrepancy. Different from the current study, in the previous work (2) catecholamine synthesis would be reduced in both PVN-projecting and nonprojecting nTS neurons, so off-setting effects could occur. Also, in the previous study catecholamine synthesis was inhibited, but the neurons remained viable, and since catecholaminergic neurons utilize cotransmitters such as glutamate (18), a cotransmitter could be responsible for maintaining basal respiration. Finally, in the current study, PVN-projecting catecholamine neurons at sites distinct from nTS (e.g., VLM and possibly other regions, such as the locus coeruleus) were also lesioned, and these may contribute to basal effects on breathing frequency. Future experiments are required to confirm the role of specific PVN-projecting catecholaminergic nuclei. In contrast to frequency, lesion of PVN-projecting catecholaminergic neurons had no effect on resting tidal volume, suggesting that the neurocircuitry controlling basal tidal volume (including pontine regions and lung afferents) has distinct inputs and neurocircuitry that may not require catecholaminergic projections to PVN.
Different from ventilatory effects, lesions of PVN-projecting catecholaminergic neurons increased resting MAP. Several mechanisms may be responsible. Catecholaminergic cells could contribute to tonic inhibition of PVN neurons with excitatory projections to cardiorespiratory brain regions such as the RVLM or spinal cord. Decreasing the number of catecholaminergic cells in the VLM likely does not contribute, as RVLM C1 cell activation increases pressure (18), and other populations of A1 and C1 cells influence primarily neuroendocrine responses (18, 55). However, loss of PVN-projecting noradrenergic neurons in nTS (A2 neurons) could be responsible for elevated arterial pressure. Although inhibiting nTS catecholamine synthesis does not alter resting pressure (2), overall selective depletion of nTS catecholamine cells leads to hypertension for several days, followed by marked chronic lability of arterial pressure (56). Furthermore, A2 lesions attenuate reflex bradycardic responses to pressor stimuli (58), indicating the importance of A2 neurons in arterial baroreflex function. Taken together, previous and current data indicate that catecholaminergic inputs to the PVN contribute to baseline breathing and blood pressure. Additional studies are required to determine specific mechanisms contributing to blood pressure effects and whether separate populations of PVN-projecting catecholaminergic neurons affect basal ventilatory and cardiovascular function.
We have shown that hypoxia produces significant activation of PVN-projecting catecholaminergic neurons in both nTS and VLM, particularly at greater hypoxia intensities (24, 26). However, the function of these projections was not determined. A major finding of the present study is that lesioning PVN-projecting catecholaminergic neurons impaired respiratory responses to peripheral chemoreflex activation. Importantly, blunted ventilation was associated with reduced oxygen saturation suggesting an impaired ability to compensate for hypoxic challenges. Furthermore, at a given oxygen saturation, ventilatory responses were markedly attenuated in DSAP compared with control rats. Together, these data demonstrate a crucial role for catecholaminergic inputs to PVN in ventilatory responses to hypoxia. It is possible that these catecholaminergic neurons also could send collateral projections to brain regions other than PVN. A portion of C1 neurons is highly collateralized (18), although most nTS neurons, including catecholaminergic neurons, project primarily to only one region (21). Given the importance of the PVN in chemoreflex function (42, 47), it is likely that catecholaminergic projections to the PVN provide a specific pathway by which chemoreflex information is integrated.
The current study did not determine specifically which catecholaminergic PVN inputs are necessary for hypoxic ventilatory responses. Although most CNS catecholaminergic cell groups are linked to cardiorespiratory regulation (5, 18), the majority of catecholamine inputs to PVN arise from VLM and nTS (9, 44, 52). Catecholaminergic VLM neurons project heavily to and influence magnocellular vasopressinergic and oxytocinergic neurons and parvocellular CRH neurons in the PVN (10, 52, 55), suggesting a role in neuroendocrine function. In contrast, both nTS and VLM project to parvocellular regions that then project to cardiorespiratory nuclei (53, 57) and thus may influence cardiorespiratory responses to hypoxia or hypercapnia. The nTS receives and integrates a variety of visceral inputs, including from chemoafferents, and has reciprocal projections to cardiorespiratory brain regions in addition to PVN (1, 40). VLM C1 neurons also have multiple projections to cardiorespiratory regions and influence breathing (5, 6, 18). Thus, catecholaminergic inputs from VLM likely contribute to neuroendocrine responses to hypoxia, whereas both nTS and VLM may play a role in chemoreflex-mediated increases in ventilation. Also unknown is the primary transmitter used within the PVN by these neurons. Whereas activation of adrenergic receptors influences both neuroendocrine and presympathetic PVN neurons (8, 32, 34), catecholaminergic neurons utilize a variety of cotransmitters, including glutamate and other peptides (18). Thus, the effects of PVN-projecting catecholaminergic neurons to influence cardiorespiratory reflexes may be mediated via catecholamines themselves or cotransmitters or by an interaction among them.
Although other studies have implicated PVN-projecting neurons in hypoxia-induced responses, none have definitively shown whether catecholaminergic input is important for ventilatory responses to chemoreflex stimulation. For example, unilateral ibotenic acid lesions, including the caudal two-thirds of the VLM catecholamine cell group, significantly reduced activation of PVN vasopressin, CRH, and oxytocin cell types following hypoxia (55). However, these lesions were not specific to catecholamine neurons, and ventilatory responses were not examined. Knockdown of overall catecholamine synthesis in nTS did not alter basal respiratory frequency but reduced ventilatory and cardiovascular responses to chronic intermittent hypoxia (2); neither direct PVN projections nor acute hypoxic responses were evaluated. Also, although blockade of adrenergic receptors in the PVN blunts cardiovascular responses and vasopressin release in response to carotid chemoreceptor stimulation in spinally transected rats (32), and adrenergic activation increases the excitability of spinally projecting PVN neurons (8, 34), neither the source of adrenergic input nor the ventilatory responses were addressed. The present work shows for the first time that catecholaminergic inputs to PVN contribute to respiratory responses to chemoreflex activation.
Augmented breaths increase in frequency during hypoxia (6, 39) and are thought to limit atelectasis and the resulting hypoventilated regions of the lung (41). Therefore, occasional augmented breaths are important for effective lung function. Interestingly, PVN activity changes dynamically during phasic respiratory events (31) and may contribute to the progression of augmented breaths. In the current study, lesions of PVN-projecting catecholaminergic neurons decreased frequency and amplitude of augmented breaths during progressive hypoxia, suggesting that catecholaminergic inputs and the PVN are necessary for these breaths. Thus, catecholaminergic projections to PVN may be important to prevent transient respiratory disturbances at the level of the lungs during hypoxia.
As described previously (24, 37), acute hypoxia decreases MAP, which progresses with hypoxia intensity. Catecholamine neurons projecting to the PVN are activated by cardiovascular challenges, including hypovolemia and osmotic stimuli (12, 24, 26). However, PVN involvement in cardiovascular adaptations specifically to hypoxia has not been evaluated thoroughly. In the present study, whereas baseline MAP was elevated following lesions of PVN-projecting catecholaminergic neurons, control and lesioned rats exhibited similar decreases in pressure to hypoxia, suggesting that the depressor response to hypoxia is independent of PVN-projecting catecholaminergic neurons. Interestingly, however, tachycardia during hypoxia was observed only in control animals, suggesting that adrenergic input to PVN contributes to certain cardiovascular responses to hypoxia. Overall, novel results from this study indicate that PVN-projecting catecholaminergic neurons may influence not only respiratory but also cardiovascular responses to hypoxia.
Regarding central chemoreflexes, a widely distributed loss of catecholaminergic neurons decreases breathing frequency and blunts responses to central chemoreceptor stimulation (33, 59). Although global PVN inhibition using lidocaine does not alter cardiorespiratory responses to central chemoreflex activation with 10% CO2 (47), PVN neurons (including vasopressin and oxytocin neurons projecting to RVLM and spinal cord) are activated by hypercapnia (11, 20, 23, 36). Novel results from the current study indicate that lesions of PVN-projecting catecholaminergic neurons significantly attenuated breathing frequency, tidal volume, and minute ventilation during systemic hypercapnia (5% CO2) in conscious rats. This was most likely due to activation of central chemoreceptors, as hypercapnia occurred in the face of hyperoxia, which minimizes peripheral chemoreflex inputs. Thus, data demonstrate that catecholaminergic neurons participate in ventilatory responses to central chemoreceptor stimulation and extend previous work to indicate that catecholaminergic projections to the PVN may be particularly important. Collectively, the present and previous findings suggest that the CO2 chemosensory system is composed of an interconnected network that influences autonomic, endocrine, and behavioral responses. Our findings demonstrate for the first time that catecholaminergic input to the PVN is part of the neurocircuitry producing respiratory responses to hypercapnia.
Perspectives
These experiments demonstrate that catecholaminergic neurons that project to the PVN are essential for the full extent of ventilatory responses to hypercapnia and hypoxia. The importance of these neurons increases at more severe intensities of hypoxia; for example, lesion reduced peak minute ventilation by more than 50% during exposure to 8% O2. This effect is functionally significant in that blunted hypoxic ventilatory responses were associated with greater decreases in O2 saturation.
Brainstem catecholaminergic neurons respond to a variety of cardiorespiratory stresses and are highly interconnected (18). Catecholaminergic neurons in nTS, CVLM, RVLM, A5, and LC are activated by hypoxia, and widespread depletion of catecholaminergic neurons diminishes responses to hypercapnia. Thus, catecholaminergic neurons, independently or together, may form a network integrating respiratory, cardiovascular, and neuroendocrine responses to cardiorespiratory and other stressors (18). The current work indicates that this network likely involves projections to the PVN from the nTS and VLM. Mechanisms within the PVN and efferent pathways from the PVN that mediate cardiorespiratory responses remain to be determined.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-98602 (to E. M. Hasser, C. M. Heesch, and D. D. Kline).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.L.K., D.D.K., C.M.H., and E.M.H. conception and design of research; T.L.K. and B.C.R. performed experiments; T.L.K., B.C.R., and E.M.H. analyzed data; T.L.K., B.C.R., and E.M.H. interpreted results of experiments; T.L.K. and B.C.R. prepared figures; T.L.K., B.C.R., and E.M.H. drafted manuscript; T.L.K., B.C.R., D.D.K., C.M.H., and E.M.H. edited and revised manuscript; T.L.K., B.C.R., D.D.K., C.M.H., and E.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We wish to acknowledge the outstanding technical expertise of Sarah A. Friskey.
REFERENCES
- 1.Andresen MC, Kunze DL. Nucleus tractus solitarius - gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Bathina CS, Rajulapati A, Franzke M, Yamamoto K, Cunningham JT, Mifflin S. Knockdown of tyrosine hydroxylase in the nucleus of the solitary tract reduces elevated blood pressure during chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1031–R1039, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res 857: 30–40, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience 156: 1093–1102, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochorishvili G, Nguyen T, Coates MB, Viar KE, Stornetta RL, Guyenet PG. The orexinergic neurons receive synaptic input from C1 cells in rats. J Comp Neurol 522: 3834–3846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke PG, Abbott G, Coates MB, Viar KE, Stornetta RL, Guyenet PG. Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats. Am J Respir Crit Care Med 190: 1301–1310, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol 348: 433–460, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Li DP, Pan HL. Presynaptic a1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther 316: 733–742, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham ET Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 292: 651–667, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham ET Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274: 60–76, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Dillon GH, Waldrop TG. In vitro responses of caudal hypothalamic neurons to hypoxia and hypercapnia. Neuroscience 51: 941–950, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Dinh TT, Flynn FW, Ritter S. Hypotensive hypovolemia and hypoglycemia activate different hindbrain catecholamine neurons with projections to the hypothalamus. Am J Physiol Regul Integr Comp Physiol 291: R870–R879, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Estacio MA, Tsukamura H, Reyes BA, Uenoyama Y, I'anson H, Maeda K. Involvement of brainstem catecholaminergic inputs to the hypothalamic paraventricular nucleus in estrogen receptor alpha expression in this nucleus during different stress conditions in female rats. Endocrinology 145: 4917–4926, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci 39: 1903–1911, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsling ML, Aziz LA. Release of vasopressin in response to hypoxia and the effect of aminergic and opioid antagonists. J Endocrinol 99: 77–86, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol 121: 147–162, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol 305: R187–R204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haselton JR, Guyenet PG. Ascending collaterals of medullary barosensitive neurons and C1 cells in rats. Am J Physiol Regul Integr Comp Physiol 258: R1051–R1063, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol 105: 35–45, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonoic targets in the rat brain. Exp Neurol 198: 539–551, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hirooka Y, Polson JW, Potts PD, Dampney RAL. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience 80: 1209–1224, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Kc P, Haxhiu MA, Tolentino-Silva FP, Mingfei W, Trouth CV, Mack SO. Paraventricular vasopressin-containing neurons project to brain stem and spinal cord respiratory-related sites. Respir Physiol Neurobiol 133: 75–88, 2002. [DOI] [PubMed] [Google Scholar]
- 24.King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 302: R1219–R1232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King TL, Heesch CM, Ruyle BC, Kline DD and Hasser EM. Catecholaminergic neurons projecting to the paraventricular nucleus (PVN) of the hypothalamus are essential for adjustments to respiratory challenges. FASEB J 27: 697, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King TL, Kline DD, Ruyle BC, Heesch CM, Hasser EM. Acute systemic hypoxia activates hypothalamic paraventricular nucleus-projecting catecholaminergic neurons in the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 305: R1112–R1123, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline DD, Buniel MC, Glazebrook P, Peng YJ, Ramirez-Navarro A, Prabhakar NR, Kunze DL. Kv1.1 deletion augments the afferent hypoxic chemosensory pathway and respiration. J Neurosci 25: 3389–3399, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kline DD, Yang T, Huang PL, Prabhakar NR. Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. J Physiol 511: 273–287, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 270: R1273–R1278, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Kristensen MP, Poe GR, Rector DM, Harper RM. Activity changes of the cat paraventricular hypothalamus during phasic respiratory events. Neuroscience 80: 811–819, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Kubo T, Yanagihara Y, Yamaguchi H, Fukumori R. Excitatory amino acid receptors in the paraventricular hypothalamic nucleus mediate pressor response induced by carotid body chemoreceptor stimulation in rats. Clin Exp Hypertens 19: 1117–1134, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol 570: 385–396, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li DP, Atnip LM, Chen SR, Pan HL. Regulation of synaptic inputs to paraventricular-spinal output neurons by alpha2 adrenergic receptors. J Neurophysiol 93: 393–402, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2328–H2336, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol (1985) 92: 826–834, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Marshall JM, Metcalfe JD. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol 407: 385–403, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins-Pinge MC, Mueller PJ, Foley CM, Heesch CM, Hasser EM. Regulation of arterial pressure by the paraventricular nucleus in conscious rats: interactions among glutamate, GABA, and nitric oxide. Front Physiol 3: 490, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCutcheon FH. The mammalian breathing mechanism. J Cell Physiol 37: 447–476, 1951. [DOI] [PubMed] [Google Scholar]
- 40.Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 263: R368–R375, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Nicholas TE, Power JH, Barr HA. The pulmonary consequences of a deep breath. Respir Physiol 49: 315–324, 1982. [DOI] [PubMed] [Google Scholar]
- 42.Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the presser response to chemoreflex activation in awake rats. Brain Res 895: 167–172, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Petrov T, Krukoff TL, Jhamandas JH. Branching projections of catecholaminergic brainstem neurons to the paraventricular hypothalamic nucleus and the central nucleus of the amygdala in the rat. Brain Res 609: 81–92, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Pickel VM, Joh TH, Reis DJ. Immunohistochemical localization of tyrosine hydroxylase in brain by light and electron microscopy. Brain Res 85: 295–300, 1975. [DOI] [PubMed] [Google Scholar]
- 46.Picklo MJ, Wiley RG, Lappi DA, Robertson D. Noradrenergic lesioning with an anti-dopamine beta-hydroxylase immunotoxin. Brain Res 666: 195–200, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol 289: R789–R797, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Reis DJ, Granata AR, Joh TH, Ross CA, Ruggiero DA, Park DH. Brain stem catecholamine mechanisms in tonic and reflex control of blood pressure. Hypertension 6: II7–II15, 1984. [DOI] [PubMed] [Google Scholar]
- 49.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 432: 197–216, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Romano A, Potes CS, Tempesta B, Cassano T, Cuomo V, Lutz T, Gaetani S. Hindbrain noradrenergic input to the hypothalamic PVN mediates the activation of oxytocinergic neurons induced by the satiety factor oleoylethanolamide. Am J Physiol Endocrinol Metab 305: E1266–E1273, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Sapru HN. Carotid chemoreflex. Neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996. [PubMed] [Google Scholar]
- 52.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257: 275–325, 1982. [DOI] [PubMed] [Google Scholar]
- 53.Sawchenko PE, Swanson LW. The organization and biochemical specificity of afferent projections to the paraventricular and supraoptic nuclei. Prog Brain Res 60: 19–29, 1983. [DOI] [PubMed] [Google Scholar]
- 54.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci 15: 7979–7988, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder DW, Nathan MA, Reis DJ. Chronic lability of arterial pressure produced by selective destruction of the catecholamine innervation of the nucleus tractus solitarii in the rat. Circ Res 43: 662–671, 1978. [DOI] [PubMed] [Google Scholar]
- 57.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980. [DOI] [PubMed] [Google Scholar]
- 58.Talman WT, Snyder D, Reis DJ. Chronic lability of arterial pressure produced by destruction of A2 catecholaminergic neurons in rat brainstem. Circ Res 46: 842–853, 1980. [DOI] [PubMed] [Google Scholar]
- 59.Viemari JC, Bevengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J Neurosci 24: 928–937, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiley RG, Kline IVRH. Neuronal lesioning with axonally transported toxins. J Neurosci Methods 103: 73–82, 2000. [DOI] [PubMed] [Google Scholar]