Abstract
Context:
It is yet unknown how similar women's hormone levels are during successive pregnancies, and very little is known about the degree to which siblings experience similar prenatal environments. Given the importance of understanding how women's reproductive life histories exert cumulative effects on health via hormone exposure, and the importance of understanding how fetal programming via endocrine signaling affects sibling trait concordance, here, we address this important lacuna in the literature.
Objective:
This study aimed to investigate how consistent women's hormone profiles are across two successive pregnancies.
Design and Main Outcome Measures:
This longitudinal, prospective study followed a cohort of 28 women across two pregnancies (PREG 1 and PREG 2). Women's circulating hormone levels were assessed from blood samples at 25, 31, and 37 weeks' gestation for adrenocorticotropic hormone (ACTH), placental corticotropin-releasing hormone (pCRH), cortisol, estradiol, and progesterone. ACTH and cortisol levels were assessed 3 months postpartum. Research questions include: Are hormone levels in PREG 2 significantly different from levels in PREG 1? What proportion of variance in PREG 2 hormone levels is attributable to variance in PREG 1 levels? Are hormone levels more stable between PREG 1 and PREG 2 compared with postpartum phases following these pregnancies? Is pCRH, which is completely placentally derived, less similar than other hormones across successive pregnancies?
Participants and Setting:
Pregnant women attended study visits at a university psychobiology laboratory in Southern California.
Results and Conclusions:
Comparisons of hormone concentrations across women's successive pregnancies via paired t test revealed substantial consistency from one pregnancy to another, with only significant differences between pregnancies for pCRH. Regressions revealed substantial predictability from one pregnancy to another, with between 17–56% of PREG 2 variances accounted for by PREG 1 values. Women exhibited lower degrees of consistency and predictability in hormone levels across postpartum phases compared with gestational concentrations. This is the first study to describe maternal and placental hormone levels across successive pregnancies.
It is unknown how similar a woman's hormone levels during one pregnancy are to the same woman's hormone levels during a subsequent pregnancy. The extent to which hormone concentrations during pregnancy are variable within a woman's life span may be influenced by maternal and fetal physiology and genetics, as well as the mother's environment, psychology, and behavior. Circulating hormone concentrations during pregnancy are of major interest for understanding how gestational endocrinology affects life span health and development of both mother and child. Previous studies have estimated cumulative hormone exposures based upon reproductive life-history patterns for the purpose of understanding how the endocrinology of female reproductive physiology exerts long-term effects on women's health (1–4), but it remains unknown whether hormone exposures from different pregnancies across a woman's life span exert equivalent effects. In addition, many studies compare monozygotic twins, dizygotic twins, and sibling pairs to determine the contributions of genetics, intrauterine environment, and postnatal environment to phenotype (5). These comparisons assume highly variable intrauterine conditions in different pregnancies of the same mother, and yet the degree of similarity between siblings' prenatal environments remains unknown.
The hormones of the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-ovarian (HPO) axes are present at the highest concentrations of the female life span during the phase of pregnancy (6, 7). For this reason, in conjunction with the observations that HPA and HPO hormones have been broadly implicated in maternal health (1–4) and as effectors of fetal programming (8–10), this study focuses on HPA hormones adrenocorticotropic hormone (ACTH), corticotropin-releasing hormone (CRH), and cortisol, and HPO hormones estradiol and progesterone. We measured mean hormone concentrations across mid to late pregnancy in two successive pregnancies for each woman (hereafter PREG 1 and PREG 2), in addition to hormone concentrations at each timepoint. Our research questions can be summarized as the following. 1) Are hormone levels in PREG 2 significantly different from hormone levels in PREG 1? 2) What proportion of variance in PREG 2 hormone levels is attributable to variance in PREG 1 hormone levels? We also address the issue of whether pregnancy represents a phase of particularly consistent hormone levels across the life span, compared with the nonpregnant state: 3) Are hormone levels more stable between PREG 1 and PREG 2 compared with the postpartum phases following PREG 1 and PREG 2? Furthermore, we predict that circulating CRH, which derives from the placenta, should exhibit less predictability in levels across successive pregnancies compared with the other hormones, which derive at least partly from maternal organs, because different fetuses are semiallogeneic to the mother. These differences in genetics could contribute to differences in phenotypes and adaptive strategies. This prompts our final research question: 4) Is placental CRH (pCRH) less similar than the other hormones across PREG 1 and PREG 2?
Materials and Methods
Cohort and procedures
Participants were women involved in a larger, prospective, longitudinal study of gestational and postnatal psychobiology at a large university medical center in Southern California. Women were recruited during their first trimester of pregnancy based on the following criteria: singleton pregnancy, over age 18 years, English speaking, nonsmoking, absence of any medical condition that could dysregulate neuroendocrine function. The subset of the larger cohort analyzed in this study were selected because they enrolled in the study twice and were included only if they attended all eight study visits relevant to our analyses, which occurred at 24–26, 30–32, and 36–38 weeks' gestation as well as 12–14 weeks postpartum, and then at the same timepoints in a subsequent pregnancy (Table 1). A blood sample was obtained at each study visit. Protocols were approved by institutional review boards of participating institutions, and written informed consent was obtained from all women before participation.
Table 1.
Cohort Descriptive Statistics
| Characteristic | Pregnancy 1 | Pregnancy 2 |
|---|---|---|
| Maternal age at delivery, mean (sd), y | 30.6 (4.5) | 32.6 (4.5) |
| Parity, frequency (%) | ||
| 0 | 19 (67.9) | n/a |
| 1 | 6 (21.4) | 18 (64.3) |
| 2 | 1 (3.6) | 7 (25.0) |
| 3 | 2 (7.1) | 1 (3.6) |
| 4 | 0 (0) | 2 (7.1) |
| No. of obstetric risk factors, frequency (%) | ||
| 0 | 22 (78.6) | 19 (67.9) |
| 1 | 5 (17.9) | 8 (28.6) |
| 2 | 1 (3.6) | 1 (3.6) |
| Baby's sex | ||
| Female | 13 (46.4) | 14 (50.0) |
| Male | 15 (53.6) | 14 (50.0) |
| Gestational age at birth, mean (sd), wk | 39.5 (1.2) | 39.2 (1.2) |
| Birth weight percentile by sex, mean (sd) | 51.9 (27.4) | 55.1 (29.6) |
| Study visit 24–26 wk gestation, mean (sd) | 25 (1.00) | 26 (0.93) |
| Study visit 30–32 wk gestation, mean (sd) | 31 (0.69) | 31 (0.80) |
| Study visit 36–38 wk gestation, mean (sd) | 37 (0.65) | 37 (0.70) |
| Study visit 12–14 wk postpartum, mean (sd) | 13 (1.10) | 13 (1.00) |
| Maternal ethnicity, frequency (%) | ||
| White, European, North African, Middle Eastern | 15 (53.6) | |
| Hispanic White | 7 (25.0) | |
| Multiethnic | 3 (10.7) | |
| Asian | 2 (7.1) | |
| African-American, Black | 1 (3.6) | |
| Maternal education, frequency (%) | ||
| Some college, vocational, or AA degree | 10 (35.7) | |
| Bachelor's degree | 10 (35.7) | |
| Graduate degree | 8 (28.6) | |
| Total household income before taxes at pregnancy 1, 15 wk gestation, mean (sd), US$ | 57 679.0 (30 738.9) | |
| Time between pregnancy 1 delivery and pregnancy 2 conception, mean (sd), d | 475.2 (198.9) | |
| Are there any parous events between pregnancies 1 and 2? frequency (%) | ||
| No | 27 (96.4) | |
| Yes | 1 (3.6) | |
| Are there any gravid events between pregnancies 1 and 2? frequency (%) | ||
| No | 22 (78.6) | |
| Yes | 6 (21.4) | |
| Baby sex concordance, frequency (%) | ||
| Female, female | 8 (28.6) | |
| Female, male | 7 (25.0) | |
| Male, female | 6 (21.4) | |
| Male, male | 7 (25.0) | |
| Breastfeeding 3-mo postpartum, frequency (%) | ||
| Pregnancy 1 | 22 (79.0) | |
| Pregnancy 2 | 22 (79.0) | |
| Both pregnancies | 17 (61.0) | |
| Menstruation recommencement 3-mo postpartum, frequency (%) | ||
| Pregnancy 1 | 20 (71.0) | |
| Pregnancy 2 | 15 (54.0) | |
| Both pregnancies | 13 (46) | |
| Hormone contraceptives 3-mo postpartum, frequency (%) | ||
| Pregnancy 1 | 7 (25.0) | |
| Pregnancy 2 | 3 (11.0) | |
| Both pregnancies | 3 (11.0) | |
Abbreviation: AA, Associate's degree.
Demographic and obstetric description of cohort (n = 28).
Endocrine measures
Blood draw occurred in the afternoon. Two 10-mL samples were withdrawn by antecubital venipuncture into EDTA-treated (purple top) vacutainers for plasma analysis, which were chilled on ice immediately, and red top vacutainers for serum analysis, which sat at room temperature until clotted (vacutainers: Becton Dickinson and Company). Blood samples in purple top vacutainers were decanted into polypropylene tubes, and 500-kallikrein inhibitor units/mL of aprotinin (Sigma-Aldrich Corp) were added. All samples were centrifuged at 2000 × g for 15-minutes, and then stored at −70°C until assaying.
Plasma ACTH levels were determined by solid-phase two-site immunoradiometric assay using human ACTH antibodies (Nichols Institute Diagnostics). Plasma samples (200 μL) combined with ACTH-labeled antibody (100 μL) and a coated bead were incubated at room temperature, and the bound radiolabeled antibody complex was quantified using a gamma scintillation counter (Isoflex, ICN Biomedical) following standard procedures [as described elsewhere (11)]. The assay has nonsignificant cross reactivity with α-endorphin and ACTH fragments. Intra-assay and interassay coefficients of variation (CV) were 4.4 and 10.8%, respectively, with a minimum detectable level of 1.0 pg/mL.
Plasma cortisol levels were ascertained by a competitive binding solid phase ELISA (IBL America). Plasma samples (25 μL) along with conjugated enzyme (200 μL) were added to the antibody-coated microtiter wells, and standard procedures were followed [as described elsewhere (11, 12)]. The absorbance units were measured at 450 nm within 10 minutes of adding stop solution. The assay has less than 9% cross reactivity with progesterone and less than 2% cross reactivity with five other naturally occurring steroids (testosterone, estradiol, estrone, estriol, aldosterone). Intra-assay and interassay CV were each less than 8%, with a minimum detectable level of 0.25 μg/dL.
Plasma CRH levels were determined by RIA (Bachem Peninsula Laboratories) following standard procedures. Plasma samples (1–2 mL) were extracted with 3 volumes of ice-cold methanol, mixed, incubated, and centrifuged. The pellets were washed with methanol, and the combined supernatants were dried down in a concentrator (SpeedVac: Savant Instruments). Labeled and unlabeled CRH samples were collected by immunoprecipitation with goat antirabbit IgG serum and normal rabbit serum, and centrifuged again. The aspirated pellets were quantified with a gamma scintillation counter. For further methodologic details see Glynn and Sandman (11). The assay has undetectable cross reactivity for human ACTH. Intra-assay and interassay CV were 5 and 15%, respectively, with a minimum detectable level of 2.04 pg/mL.
Serum 17β-estradiol levels were ascertained by microtiter well competitive binding enzyme immunoassay (ELISA, IBL America) following standard procedures [as described elsewhere (12)]. Diluted samples (25 μL) were incubated with conjugated enzyme (200 μL) in each well, and substrate reagent (100 μL) was added and incubated. Enzymatic reaction was halted with stop reagent (50 μL), and within 10 minutes, absorbance readings were taken at 450 nm. The assay has less than 0.2% cross reactivity with estriol and estrone, and nondetectable cross reactivity with 17α-estradiol and 25 other naturally occurring steroids. Interassay and intra-assay CV were less than 10 and 7%, respectively, with a minimum detectable level of 9.7 pg/mL.
Serum progesterone levels were determined by microtiter-well competitive binding enzyme immunoassay (ELISA, IBL America) following standard procedures. Diluted samples (25 μL) were incubated with conjugated enzyme (200 μL) in each well, and substrate reagent (200 μL) was added and incubated. Within 10 minutes of adding stop solution (50 μL), absorbance readings were taken at 450 nm. The assay has 1.1% cross reactivity with 11-desoxycorticosterone, less than 0.4% cross reactivity with pregnenolone, 17α-OH progesterone, and less than 0.1% cross-reactivity with corticosterone, estriol, 17β-estradiol, cortisol, and three other naturally occurring steroids. Inter- and intra-assay coefficients of variance were less than 10 and 7%, respectively, with a minimum detectable level of 0.045 ng/mL.
Data analysis plan
The timepoints of 25, 31, and 37 weeks' gestation were selected to capture the window when hormone levels are highest. In addition to each individual timepoint, the means of the three timepoints were also investigated to minimize bias from the timing of any individual study visit and eliminate variability based on acute fluctuations. Thereby, this method optimized accuracy in reflecting hormone levels across the course of mid to late pregnancy.
Research question 1: Are hormone levels in PREG 2 significantly different from hormone levels in PREG 1?
For mean values and for each timepoint individually, we evaluated the significance of the difference in PREG 1 and PREG 2 hormone levels by paired t test, and we assessed the magnitude of change in hormone concentrations by computing PREG 2 levels as a function of PREG 1 levels.
Research question 2: What proportion of variance in PREG 2 hormone levels is attributable to variance in PREG 1 hormone levels?
First, we used regression models to investigate the following relationships to evaluate whether potential covariates should be included in models: maternal age, gestational age, and time of day at sample collection (“time of collection”). Time of collection was significantly related to cortisol concentrations at 25 and 31 weeks' gestation (Supplemental Table 1). Consequently, we residualized all cortisol values by time of collection and used these residuals in all subsequent analyses. There were no other significant associations with covariates.
For regression models for each hormone, the independent (PREG 1) and dependent (PREG 2) variables used were hormone concentrations at each of three timepoints, as well as the mean across the three timepoints. In addition, we investigated whether the changes in hormone levels from 25 to 37 weeks' gestation were consistent between PREG 1 and PREG 2. Each variable was transformed to improve distribution when necessary. Gaussian distribution was assessed by visual inspection of histograms and Shapiro-Wilk P > .10 (Supplemental Table 2). For each hormone, cases with missing data at any timepoint for either pregnancy were excluded from all models for that hormone, resulting in sample sizes of 19 to 21 women (Table 2).
Table 2.
Descriptive Statistics of Hormone Concentrations
| Hormone | Statistic | PREG 1 | PREG 2 | Postpartum PREG 1 (n = 24) | Postpartum PREG 2 (n = 24) |
|---|---|---|---|---|---|
| ACTH, pg/mL (n = 21) | |||||
| Mean | 44.09 | 36.57 | 22.68 | 16.01 | |
| Range | 21.19–129.15 | 18.93–77.3 | 6.94–59.42 | 4.06031.90 | |
| se. mean | 5.36 | 3.89 | 2.50 | 1.61 | |
| CI mean 95% | 11.17 | 8.10 | 5.17 | 3.33 | |
| sd | 24.55 | 17.80 | 12.24 | 7.88 | |
| Cortisol, μg/mL (n = 19) | |||||
| Mean | 20.85 | 21.75 | 6.39 | 7.07 | |
| Range | 14.54–39.25 | 16.57–30.34 | 1.98–15.70 | 3.13–22.26 | |
| se. mean | 1.23 | 0.84 | 0.66 | 0.91 | |
| CI mean 95% | 2.59 | 1.77 | 1.37 | 1.89 | |
| sd | 5.38 | 3.68 | 3.25 | 4.46 | |
| pCRH, pg/mL (n = 19) | |||||
| Mean | 387.97 | 314.31 | |||
| Range | 115.37–657.83 | 84.07–587.97 | |||
| se. mean | 37.76 | 35.52 | |||
| CI mean 95% | 79.33 | 74.63 | |||
| sd | 164.58 | 154.83 | |||
| Estradiol pg/mL (n = 19) | |||||
| Mean | 4930.30 | 4927.02 | |||
| Range | 3230.37–6054.08 | 3403.98–7039.38 | |||
| se. mean | 181.85 | 244.54 | |||
| CI mean 95% | 382.06 | 513.76 | |||
| sd | 792.67 | 1065.92 | |||
| Progesterone, ng/mL (n = 20) | |||||
| Mean | 88.27 | 85.76 | |||
| Range | 43.18–189.77 | 42.18–131.96 | |||
| se. mean | 7.34 | 5.24 | |||
| CI mean 95% | 15.36 | 10.98 | |||
| sd | 32.83 | 23.46 |
Abbreviation: CI, confidence interval.
Each hormone refers to the mean of hormone level measurements at 25, 31, and 37 weeks' gestation for PREG 1 and PREG 2. Postpartum values refer to hormone levels measured at one time point for each pregnancy 3-month delivery. Total cohort size was n = 28. .
Using visual inspection of curves fitted by locally estimated scatterplot smoothing (LOESS) and ANOVA comparisons, it was determined that linear models were the best fit for the data. Linear regression models measured the statistical reliance of PREG 2 hormone concentrations upon PREG 1 hormone concentrations. We investigated the following covariates as interaction terms in models for all hormones: parity, gravidity, time between pregnancies (days between PREG 1 delivery and PREG 2 conception), and child's sex concordance for the two pregnancies. No interaction terms contributed significant effects for any model. To optimize accuracy of models, we omitted a small number of cases that had excessive leverage, influence, or outlier values, according to conventional criteria (13) (Supplemental Table 3). Plots of residuals vs fitted values revealed no indication of heteroscedasticity or nonlinearity.
Research question 3: Are hormone levels more stable between PREG 1 and PREG 2 compared with the postpartum phases following PREG 1 and PREG 2?
We assessed how alike women's hormone concentrations were in the same cohort of women 3 months after PREG 1 and PREG 2 deliveries. We only investigated ACTH and cortisol because CRH is not detectable in circulation in nonpregnant women, and estradiol and progesterone would only have been relevant to measure in women who were not using hormonal contraception, leaving an insufficient sample size (N = 9). First, we determined by linear regression that time of collection was significantly related to postpartum cortisol for both PREG 1 and PREG 2, and unrelated to ACTH (Supplemental Table 1). Consequently, we used residualized postpartum cortisol values in all subsequent models. Next, we determined that neither postpartum ACTH nor cortisol concentrations were related to number of days since delivery. Final linear regression models adjusted for breastfeeding, hormonal contraception, and whether menstrual cycling had recommenced (Table 1). Lastly, we optimized the accuracy of our models by excluding women whose hormone concentrations had excessive leverage, influence, or outlier values (Supplemental Table 3). These exclusions did not change statistical significance of models.
Research question 4 is based on a comparison of regression and t test results of pCRH to the other hormones. This question does not require further statistical analyses, so it is addressed in the Discussion section. All analyses were conducted using the R programming language and RStudio (Version 0.98.1091; RStudio Inc) environment for statistical computing.
Results
Cohort descriptives
The cohort included 28 women, with mean age of 30.6 years at PREG 1 delivery and 32.6 years at PREG 2 delivery, with a mean of 1.3 years between PREG 1 delivery and PREG 2 conception (Table 1). Data include 27 participants for whom PREG 2 was the directly subsequent delivery after PREG 1, and one case in which there was an interim delivery not included in the study. Also, five participants experienced a miscarriage between PREG 1 and PREG 2. At PREG 1, 68% of women were primiparous. Demographic information is presented in Table 1.
Research question 1: Are hormone levels in PREG 2 significantly different from hormone levels in PREG 1?
Hormone levels in PREG 1 and PREG 2 are described in Table 2. Comparisons of group mean hormone concentrations in PREG 1 and PREG 2 via paired t test revealed that the only significant (P < .10) difference was for pCRH (Table 3). Comparisons of each hormone's concentration at each individual timepoint via paired t test revealed that the only significant (P < .10) differences were pCRH at 31 weeks' gestation (mean of differences = 120.23 pg/mL; t (1, 18) = 2.38; P = .03) and ACTH at 37 weeks' gestation (mean of differences = 17.71 pg/mL; t (1, 20) = 2.95; P = .01).
Table 3.
Comparisons of Hormone Concentrations in PREG 1 and PREG 2
| Status | Hormone | Comparison of Means | Proportion |
|---|---|---|---|
| Paired t test of PREG 1 and PREG 2 means | Mean of (PREG 2 ÷ PREG 1) × 100 | ||
| Pregnancy | ACTH | t (20) = 1.6; P = 0.13 | 96.02% |
| pCRH | t (18) = 1.8; P = 0.08 | 91.92% | |
| Cortisol | t (18) = −0.91; P = 0.37 | 107.22%a | |
| Estradiol | t (18) = 0.01; P = 0.98 | 101.89% | |
| Progesterone | t (19) = 0.45; P = 0.66 | 101.63% | |
| Post-partum | ACTH | t (23) = 14; P = 7.8e-13 | 49.96% |
| Cortisol | t (23) = −16; P = 5.2e-14 | 59.28%a |
The Comparison of Means column lists results of paired t tests in which mean hormone levels in PREG 1 and PREG 2 were compared. P values reflect whether hormone levels in PREG 1 were significantly distinct from hormone levels in PREG 2. There are no statistically significant differences, besides pCRH [t(18) = 1.8; P = .08; mean (M) of differences = 73.67 pg/mL; M of the absolute value of differences = 142.77 pg/mL].
The Proportion column lists the means of PREG 2 hormone levels as a function of PREG 1 levels. Cortisol data were residualized by time of day at collection unless otherwise indicated.
Calculated using unresidualized cortisol values.
Also, we explored the stability of interpregnancy hormone levels by calculating PREG 2 levels as a percentage of PREG 1 levels for each hormone. Examining the means of these proportions, we found stable patterns for each hormone, reflecting the mostly-nonsignificance of interpregnancy differences. PREG 2 ACTH level was on average 4.0% lower than PREG 1 level. PREG 2 pCRH level was on average 8.1% lower than PREG 1 level. Cortisol was on average 7.2% higher in PREG 2 compared with PREG 1. Estradiol and progesterone were the most stable, less than 2% higher in PREG 2 than PREG 1 on average (Table 3).
Research question 2: What proportion of variance in PREG 2 hormone levels is attributable to variance in PREG 1 hormone levels?
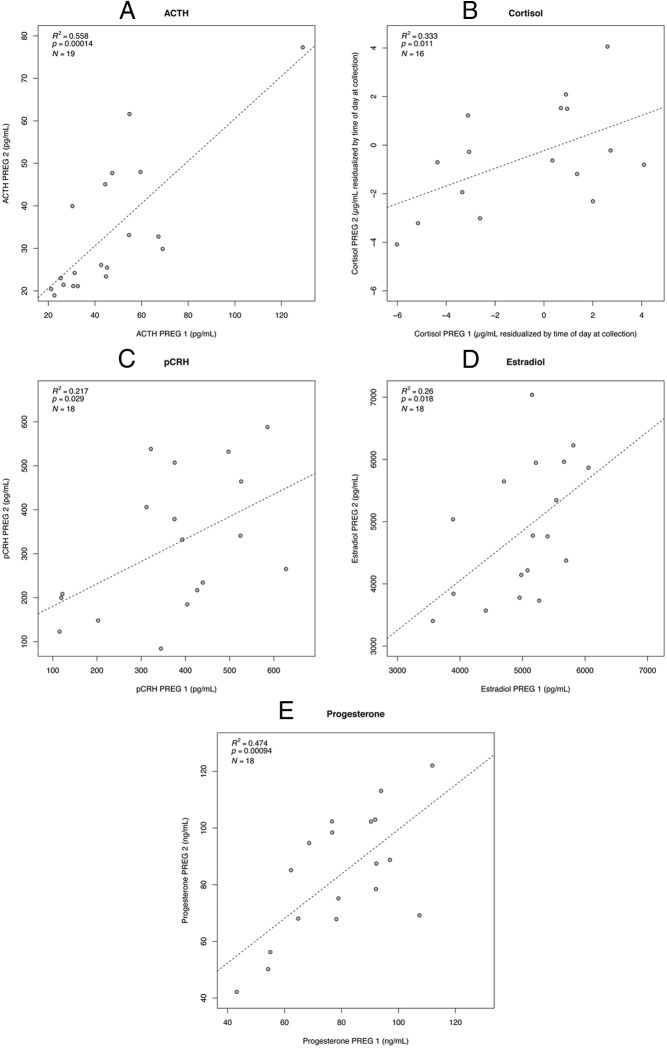
Analyzing data as mean hormone concentrations across gestation as well as individual timepoints, we find that ACTH and progesterone exhibit the most consistency across pregnancies, estradiol and cortisol are intermediate, and pCRH exhibits the least consistency. Figure 1 shows scatterplots with regression lines for PREG 1 × PREG 2 mean hormone levels. For mean hormone concentrations, ACTH exhibited the strongest effect magnitude, with 55.8% of variance in PREG 2 accounted for by values in PREG 1. For progesterone, 47.4% of variance in PREG 2 was accounted for by values in PREG 1. For cortisol it was 33.3%, and for estradiol 26.0%. The weakest association was exhibited by pCRH with 16.8% of variance in PREG 2 accounted for by values in PREG 1 (Table 4). We also report the linear regression results for each individual timepoint across the two pregnancies (Table 5), which follow a similar pattern to the mean hormone level results. ACTH and progesterone showed a significant correlation between PREG 1 and PREG 2 levels for all three timepoints. Cortisol and estradiol showed a significant correlation between PREG 1 and PREG 2 levels for two of the three timepoints. pCRH showed a significant correlation between PREG 1 and PREG 2 levels for only one timepoint, 37 weeks' gestation.
Figure 1.
Correlations between PREG 1 and PREG 2 hormone levels. Scatterplots with regression lines use untransformed data to display the relationship between means of hormone concentrations at 25, 31, and 37 weeks' gestation in PREG 1 and PREG 2. Regression results written in the corner of each plot use data transformations described in Supplemental Table 2. All cortisol values were residualized by time of day at collection (Supplemental Table 1). A, ACTH; B, cortisol; C, pCRH; D, estradiol; and E, progesterone.
Table 4.
Stability of Mean Hormone Concentrations Across Subsequent Pregnancies
| Status | Hormone | N | F-statistic | DF | Adjusted R2 | P |
|---|---|---|---|---|---|---|
| Pregnancy | ACTH | 19 | 23.75 | 1,17 | 0.558 | .00a |
| pCRH | 18 | 4.43 | 1,16 | 0.168 | .05b | |
| Cortisol | 16 | 8.50 | 1,14 | 0.333 | .01c | |
| Estradiol | 18 | 6.97 | 1,16 | 0.260 | .02c | |
| Progesterone | 18 | 16.34 | 1,16 | 0.474 | .00a | |
| Post-partum | ACTH | 20 | 0.67 | 4,15 | −0.075 | .41 (ns) |
| Cortisol | 22 | 0.68 | 4,14 | −0.077 | .29 (ns) |
Abbreviations: DF, degrees of freedom; ns, not significant.
Regression analyses measure the proportion of variance in PREG 2 hormone levels attributable to variance in PREG 1 hormone levels. Cortisol data were residualized by time of day at collection.
Postpartum models control for breastfeeding, resumption of menses, and hormonal contraceptive use. For the null postpartum models, the negative adjusted R2 values are interpretable as zero.
P < .10 are in bold. See Supplemental Tables 2 and 3 for model details.
P < .001.
P < .10.
P < .05.
P < .01.
Table 5.
Stability of Hormone Concentrations at Each Timepoint Across Subsequent Pregnancies
| Hormone | Gestation Timepoint (wk) | N | F-statistic | DF | Adjusted R2 | P |
|---|---|---|---|---|---|---|
| ACTH | 25 | 20 | 3.5 | 1,18 | 0.115 | .079a |
| 31 | 20 | 5.9 | 1,18 | 0.206 | .026b | |
| 37 | 19 | 6.4 | 1,17 | 0.231 | .022b | |
| pCRH | 25 | 19 | 0.0 | 1,17 | −0.058 | .921 |
| 31 | 19 | 1.3 | 1,17 | 0.015 | .273 | |
| 37 | 19 | 6.4 | 1,17 | 0.232 | .0213b | |
| Cortisol | 25 | 17 | 5.8 | 1,15 | 0.230 | .030b |
| 31 | 18 | 9.9 | 1,16 | 0.342 | .006c | |
| 37 | 18 | 1.6 | 1,16 | 0.032 | .228 | |
| Estradiol | 25 | 19 | 4.5 | 1,17 | 0.164 | .048b |
| 31 | 18 | 6.8 | 1,16 | 0.253 | .019b | |
| 37 | 18 | 0.0 | 1,16 | −0.061 | .877 | |
| Progesterone | 25 | 19 | 22.6 | 1,17 | 0.546 | .000d |
| 31 | 20 | 5.0 | 1,18 | 0.176 | .038b | |
| 37 | 18 | 12.4 | 1,16 | 0.401 | .003c |
Abbreviations: DF, degrees of freedom; ns, not significant.
Regression analyses measure the proportion of variance in PREG 2 hormone levels attributable to variance in PREG 1 hormone levels at each of three timepoints (25, 31, 37 weeks' gestation).
Cortisol data were residualized by time of day at collection. For null results, negative adjusted R2 values are interpretable as zero.
P < .10 are in bold. See Supplemental Tables 2 and 3 for model details.
P < .10.
P < .05.
P < .01.
P < .001.
In addition, we used linear regression to investigate whether the changes in hormone levels from 25 to 37 weeks' gestation were consistent between PREG 1 and PREG 2. Only pCRH exhibited significant consistency in change in concentration during gestation between PREG 1 and PREG 2 (F (1, 15) = 4.6; adjusted R2 = 0.18; P = .05), whereas ACTH, cortisol (both unresidualized and residualized for time of collection), estradiol, and progesterone exhibited no significant consistency in change during gestation between PREG 1 and PREG 2 (P > .10).
Research question 3: Are hormone levels more stable between PREG 1 and PREG 2 compared with the postpartum phases following PREG 1 and PREG 2?
Comparisons of group mean hormone concentrations at 3 months postpartum via paired t test revealed significant differences in both ACTH and cortisol between PREG 1 and PREG 2 (Table 3). The changes in ACTH and cortisol levels from the postpartum phase of PREG 1 to the postpartum phase of PREG 2 were of substantially greater magnitude than the changes that occurred across the pregnancy phases. Hormone level during the PREG 2 postpartum phase as a percentage of level during the PREG 1 postpartum phase was a mean of 50.0% for ACTH and 59.3% for cortisol (Table 3). Altogether, these results reveal a low degree of stability in hormone levels across the postpartum phases of successive pregnancies.
Neither ACTH nor cortisol exhibited any significant correlation between PREG 1 postpartum and PREG 2 postpartum concentrations (Table 4). We repeated the postpartum ACTH and cortisol analyses restricting the cohort to those participants included in the pregnancy analyses, and the results remained null (ACTH: F (4, 10) = 2.87; P = .14; cortisol: F (4, 7) = 0.39; P = .40). These results reveal a low degree of predictability in hormone levels across the postpartum phases of successive pregnancies.
Discussion
Results suggest that hormones in maternal circulation during pregnancy are relatively stable from one pregnancy to another within a woman's life history. Hormones during two nonpregnant states equally separated by time seem to be far less stable by comparison. These results have important implications for gestational biology, maternal health, fetal development, and child health.
Stability of pregnancy physiology across the life span
Concentrations of HPA and HPO hormones during pregnancy are substantially greater than during the nonpregnant state. Conceivably, factors that cause fluctuations in hormone concentrations during nonpregnant phases may exert less influence during pregnancy for two reasons. Firstly, endocrine systems may be less responsive to these factors because hormone levels are held close to their physiological maximum by gestational homeostatic systems (14, 15). The physiological demands of pregnancy can act as a challenge to the somatic system, enlisting all available, relevant, maternal resources toward meeting the demands of the developing fetus (16). Circulating concentrations of hormones may be relatively stable from one pregnancy to the next because pregnancy may reveal endocrine ceiling effects, and how ceiling effects may change across the life history.
Secondly, stability of hormone levels across pregnancies may reflect desensitization of endocrine systems to external perturbation. This possibility is supported by previous evidence that imposing external stressors on pregnant women elicits a dampened physiological response compared with nonpregnant women, eg, blood pressure (17), HPA-axis activation (18), and psychological responses to stress (19, 20). Whether endocrinological inflexibility or insensitivity plays a functional role in pregnancy remains unknown. Additional research is necessary to explain the striking degree of stability we observe in hormone levels across successive pregnancies.
Previous studies of nonpregnant adults have shown low intra-individual stability of hormone levels in baseline conditions and higher stability in challenge conditions [ACTH, cortisol, lipotropic hormone (21), cortisol (22, 23), cortisol, luteinizing hormone (24)]. Our observation that postpartum (ie, baseline) ACTH and cortisol levels were inconsistent compared with the consistency during pregnancy (ie, challenge) is congruous with these previous observations.
Research question 4: Is pCRH less similar than the other hormones across PREG 1 and PREG 2?
We predicted that pCRH should be less predictable from one pregnancy to the next compared with hormones that derive, entirely or in part, from maternal organs. Circulating levels of maternal plasma CRH during pregnancy is nearly exclusively derived from the placenta (25). In support of our hypothesis, we observed pCRH to be the least predictable from PREG 1 to PREG 2 of all the hormones investigated here. Notably, CRH was the only hormone that exhibited significant difference comparing group means for PREG 1 and PREG 2.
By comparison, maternal plasma cortisol during pregnancy is exclusively derived from the maternal adrenal glands, ACTH is nearly exclusively derived from the maternal pituitary, and estradiol and progesterone reflect both maternal (ovarian) and placental secretion (6). The relative stability we observed across two successive pregnancies for these hormones could reflect stability in the mother's reproductive strategy, compared with pCRH instability reflecting variation in strategies of semiallogeneic fetuses. Possibly, pCRH could be less stable across pregnancies than other hormones with partial placental contribution because it is more sensitive to environmental conditions, which vary stochastically between subsequent pregnancies.
Previous studies
Two previous studies analyzed gestational physiology across two successive pregnancies in cohorts of women. The first study found that women exhibited intraindividual correlations in weight, body mass index, and offspring birth weight across the two pregnancies, and no association for maternal hemoglobin (26). The second study compared various aspects of gestational physiology in 106 women across two pregnancies (27). For indicators of maternal sympathetic activation, they found that maternal electrodermal activity was greater during the subsequent pregnancy, whereas respiratory sinus arrhythmia was greater during the earlier pregnancy, and no trend in directionality for maternal heart rate or respiratory period. They did not explore correlations across the two pregnancies. Similar to our results, they found no moderating effects of fetal sex concordance. Their study began the important exploration of the degree to which siblings share a prenatal environment. Our results expand this field of inquiry as the first investigation of intra-individual gestational endocrine concordance.
Implications for understanding maternal health
Knowing whether women experience similar concentrations of hormones in each of their pregnancies can improve our estimation of lifetime (cumulative) exposures to the endocrine conditions of pregnancy. Because pregnancy is characterized by the highest concentrations of glucocorticoids and gonadotropins in a woman's lifetime, and because these hormones have been implicated in disease etiology, this topic is of major interest for women's health. Glucocorticoids are involved in a wide range of immunological functions, including modulation of gene expression, suppression of certain pathways, and promotion of others (28). Cumulative exposure to high concentrations of estrogens has been positively associated with risk of reproductive cancers [breast (1), ovarian (3), and endometrial (4)], and negatively associated with risk of Alzheimer's Disease (2). Our calculations of the intra-individual stability in gestational hormone levels represent an important step in improving our estimation of the cumulative effect of reproductive life history on later-life disease risk via cumulative hormone exposure. In addition, maternal endocrine profiles during gestation have been implicated in maternal cognitive performance (12), maternal sensitivity (29), and postpartum depression (30–32). Our results contribute to a better understanding of how successive pregnancies (and postpartum phases) may influence a woman's health across her life span.
Reconceptualizing the early shared environment: Fetal programming and sibling effects
Appreciating the degree of consistency in a mother's hormone concentrations across pregnancies will improve our understanding of the underlying mechanisms involved in sibling trait concordance. Hormone exposures during the prenatal phase of life during sensitive periods moderate fetal developmental processes (8) in ways that have lifelong, often irreversible, consequences for offspring health and development (9, 10). This is part of the process of fetal programming. For certain traits, prenatal hormone exposures play a major role in shaping phenotype (9, 33–36). For such traits, two siblings exposed to similar endocrine environments in utero may exhibit trait concordance.
Until now, we did not know how similar siblings' fetal hormone exposures were, limiting our ability to draw accurate conclusions about prenatal and postnatal environmental influences on phenotypic development. Many “extended twin studies” have compared monozygotic twins, dizygotic twins, and siblings to discern the genetic, prenatal, and postnatal environment influences on a wide range of traits, eg, brain morphology (37), depression (38), drug abuse (39), cardiovascular disease risk (40), and diabetes risk (41). These study designs are based on the premise that nontwin siblings have the same genetic relatedness as dizygotic twins but experience different intrauterine environments. Thus, an underlying assumption of the study design is that within a mother, gestational physiologies during two of her pregnancies are different enough from one another to reveal the effects of prenatal programming. Maternal age, environmental circumstances that affect maternal somatic and placental function, and fetal identity differ across pregnancies. Yet, maternal identity remains consistent, maternal-placental genetics remain consistent, and fetal (and fetal-placental) genetic identity is still half of maternal origin, and, in some cases, has shared paternal genetic origin with the antecedent sibling. Therefore, some aspects of gestational biology are consistent across successive pregnancies, whereas other aspects vary, predicting some (but not total) consistency in endocrinology, as our results demonstrate. Because of the important role of hormones as effectors of fetal programming, the aspects of gestational biology that account for endocrinologic differences across successive pregnancies may promote divergent sibling phenotypic development, whereas the aspects of gestational biology that account for endocrinologic similarities across successive pregnancies may promote concordant sibling phenotypic development. In conclusion, the way we interpret comparisons of dizygotic twin vs sibling trait concordance needs to be reconsidered based on a more informed understanding of a mother's interpregnancy physiological consistency.
In addition, the correlation between fetal hormone exposure and maternal circulating hormone levels is not one to one and may vary (42, 43). Additional studies are needed to investigate differences in hormones not only in maternal circulation but also in utero across successive pregnancies.
Conclusion
We find that in this cohort, up to 56% of the variance in hormone levels in a pregnancy can be predicted from hormone levels in a previous pregnancy. This interpregnancy consistency in hormone levels is absent during the nonpregnant state. Future studies should further investigate this topic in a larger cohort. Nonetheless, our results can inform future efforts to estimate women's cumulative hormone exposures based on reproductive life-history. Also, these results suggest that a substantial portion of siblings' shared environments may be prenatal, which should alter how we interpret observations of sibling trait concordance.
Acknowledgments
We thank the families who participated in these projects. We also thank the dedicated staff at the Women and Children's Health and Well-Being project.
This work was supported by research grants from the National Institutes of Health (HD-40967, NS-41298, and MH-96889).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- CI
- confidence interval
- CV
- coefficient of variation
- DF
- degrees of freedom
- HPA
- hypothalamic-pituitary-adrenal
- HPO
- hypothalamic-pituitary-ovarian
- LOESS
- locally estimated scatterplot smoothing
- ns
- not significant
- pCRH
- placental corticotropin-releasing hormone
- PREG
- pregnancy
- SD
- standard deviation
- SE
- standard error.
References
- 1. Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003;5:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer's risk in a cohort of British women. Psychoneuroendocrinology. 2013;38:2973–2982. [DOI] [PubMed] [Google Scholar]
- 3. Adami H, Lambe M, Persson I, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344:1250–1254. [DOI] [PubMed] [Google Scholar]
- 4. Schonfeld SJ, Hartge P, Pfeiffer RM, et al. An aggregated analysis of hormonal factors and endometrial cancer risk by parity. Cancer. 2013;119:1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posthuma D, Boomsma DI. A note on the statistical power in extended twin designs. Behav Genet. 2000;30:147–158. [DOI] [PubMed] [Google Scholar]
- 6. Mesiano S. The endocrinology of human pregnancy and fetal-placental neuroendocrine development. In: Strauss JF, Barbieri R, eds. Yen and Jaffe's reproductive endocrinology: physiology, pathophysiology, and clinical management. Philadelphia: Elsevier Saunders; 2014:243–271. [Google Scholar]
- 7. Tulchinsky D, Little AB. Maternal-fetal endocrinology. 2nd ed Philadelphia: W.B. Saunders. [Google Scholar]
- 8. Glynn LM, Sandman CA. Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Dev Sci. 2012;15:601–610. [DOI] [PubMed] [Google Scholar]
- 9. Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert Rev Endocrinol Metab. 2012;7:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014;76:355–362. [DOI] [PubMed] [Google Scholar]
- 12. Glynn LM. Giving birth to a new brain: Hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010;35:1148–1155. [DOI] [PubMed] [Google Scholar]
- 13. Rahmatullah Imon A. Identifying multiple influential observations in linear regression. J Appl Stat. 2005;32:929–946. [Google Scholar]
- 14. Marcus S, Lopez JF, McDonough S, et al. Depressive symptoms during pregnancy: Impact on neuroendocrine and neonatal outcomes. Infant Behav Dev. 2011;34:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schulte H, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: Lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf). 1990;33:99–106. [DOI] [PubMed] [Google Scholar]
- 16. Williams D. Pregnancy: A stress test for life. Curr Opin Obstet Gynecol. 2003;15:465–471. [DOI] [PubMed] [Google Scholar]
- 17. Matthews KA, Rodin J. Pregnancy alters blood pressure responses to psychological and physical challenge. Psychophysiology. 1992;29:232–240. [DOI] [PubMed] [Google Scholar]
- 18. Kammerer M, Adams D, Von Castelberg B, Glover V. Pregnant women become insensitive to cold stress. BMC pregnancy and childbirth. 2002;2:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: Effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. [DOI] [PubMed] [Google Scholar]
- 20. Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. [DOI] [PubMed] [Google Scholar]
- 21. Coste J, Strauch G, Letrait M, Bertagna X. Reliability of hormonal levels for assessing the hypothalamic-pituitary-adrenocortical system in clinical pharmacology. Br J Clin Pharmacol. 1994;38:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. [DOI] [PubMed] [Google Scholar]
- 23. Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–86. [DOI] [PubMed] [Google Scholar]
- 24. Schulz P, Knabe R. Biological uniqueness and the definition of normality. Part 2—The endocrine ‘fingerprint’ of healthy adults. Med Hypotheses. 1994;42:63–68. [DOI] [PubMed] [Google Scholar]
- 25. McLean M, Smith R. Corticotropin-releasing hormone in human pregnancy and parturition. Trends Endocrinol Metab. 1999;10:174–178. [DOI] [PubMed] [Google Scholar]
- 26. Khan KS, Chien PF, Khan NB. Nutritional stress of reproduction. A cohort study over two consecutive pregnancies. Acta Obstet Gynecol Scand. 1998;77:395–401. [PubMed] [Google Scholar]
- 27. DiPietro JA, Costigan KA, Voegtline KM. Studies in fetal behavior: Revisited, renewed, and reimagined. Monogr Soc Res Child Dev. 2015;80:1–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franchimont D. Overview of the actions of glucocorticoids on the immune response: A good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–137. [DOI] [PubMed] [Google Scholar]
- 29. Fleming AS, Ruble D, Krieger H, Wong P. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm Behav. 1997;31:145–158. [DOI] [PubMed] [Google Scholar]
- 30. Yim IS, Glynn LM, Dunkel-Schetter CD, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL. Transdermal estradiol for postpartum depression: A promising treatment option. Clin Obstet Gynecol. 2009;52:516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandman C, Davis E, Buss C, Glynn L. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. [DOI] [PubMed] [Google Scholar]
- 36. Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. [DOI] [PubMed] [Google Scholar]
- 37. Baaré WF, Hulshoff Pol HE, Boomsma DI, et al. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. [DOI] [PubMed] [Google Scholar]
- 38. Boomsma DI, Beem AL, van den Berg M, et al. Netherlands twin family study of anxious depression (NETSAD). Twin Res. 2000;3:323–334. [DOI] [PubMed] [Google Scholar]
- 39. Kendler KS, Maes HH, Sundquist K, Ohlsson H, Sundquist J. Genetic and family and community environmental effects on drug abuse in adolescence: A Swedish national twin and sibling study. Am J Psychiatry. 2014. February;171(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kupper N, Willemsen G, Boomsma DI, de Geus EJ. Heritability of indices for cardiac contractility in ambulatory recordings. J Cardiovasc Electrophysiol. 2006;17:877–883. [DOI] [PubMed] [Google Scholar]
- 41. Simonis-Bik A, Eekhoff EM, Diamant M, et al. The heritability of HbA1c and fasting blood glucose in different measurement settings. Twin Res Hum Genet. 2008;11:597–602. [DOI] [PubMed] [Google Scholar]
- 42. van de Beek C, Thijssen JH, Cohen-Kettenis PT, van Goozen SH, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: What is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav. 2004;46:663–669. [DOI] [PubMed] [Google Scholar]
- 43. Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–109. [DOI] [PubMed] [Google Scholar]