Abstract
Background:
This study sought to assess the function and delivery reliability of intradermal (ID) infusion sets used with commercial insulin pumps.
Method:
Healthy subjects (n = 43) were randomized to either ID or subcutaneous (SC) arms, and received basal/bolus placebo delivery for 24 hours. Subjects received 4 of 8 infusion set combinations (ID: microneedle design A or B, with 2 pump brands [Animas or MiniMed]; SC: Teflon Quickset or steel Rapid-D, Animas pump only, with or without overtaping) and were evaluated for pump occlusion alarms, fluid leakage, pain, and tissue tolerability. A novel algorithm was developed to determine flow consistency based on fluid pressure, and the duration and occurrence rate for periods of unalarmed but interrupted flow (“silent occlusions’”) were compared.
Results:
ID delivery was successfully maintained over the 24-hour infusion period. The number of silent occlusions was lower for ID microneedle cannula design B than A (P < .01) and lower for Rapid-D SC device compared to Quick-set (P = .03). There was no significant difference in the number of occlusion alarms between the ID and SC devices with the Animas pump. However, the pumps tested with ID devices had significantly different alarm rates (MiniMed 29.5%, Animas 0%, P < .001). Leakage and tissue tolerability were comparable across devices.
Conclusion:
The ID infusion set reliably delivered diluent for an extended 24-hour period in healthy subjects and was well tolerated. Silent occlusion flow interruptions could be detected in both ID and SC infusion sets using a proprietary algorithm. This algorithm is a promising method for quantitatively evaluating infusion set flow performance.
Keywords: continuous subcutaneous insulin delivery, insulin infusion catheter sets, insulin pump occlusion, intradermal insulin delivery, silent occlusion
Intradermal (ID) delivery is an alternative method of insulin administration that may have pharmacological advantages, including faster pharmacokinetics and enhanced bioavailability, and result in tighter postprandial glucose control compared with continuous subcutaneous insulin infusion (CSII).1-4 These effects may be attributed to targeting the extensive capillary and lymphatic beds in the dermal region.4
Feasibility of continuous insulin infusion, regardless of route, relies on delivery reliability and consistency and timely detection of flow interruptions or occlusions. Occlusions are one of the most common faults associated with insulin pump therapy.5-7 Currently available pump alarms are generally not sufficiently sensitive to rapidly detect insulin flow interruptions, which if left undiagnosed, could rapidly lead to glycemic imbalance and diabetic ketoacidosis.8-11 Examination of 5 commercially available pumps during basal insulin infusion at either 0.5 or 1.0 IU/h with different infusion set lengths (2.5 or 60 cm) found minimal alarm times ranging between approximately 0.5 to 4.5 hours for high flow rate/short infusion set length situations and up to 1.5 to 9.5 hours for low flow/long infusion set situations.5 This relatively slow occlusion detection and announcement, coupled with the fact that set blockage is a frequent complication during CSII use,9 suggests that methods for evaluating infusion set flow performance during use are not well established. Previous studies5,7,9,12 have identified the potential for infusion flow interruptions that occur below the alarm threshold for various insulin infusion pumps. These unalarmed flow stoppages are termed “silent occlusions.”
The aim of this study was to investigate the feasibility, capability and reliability of a novel microneedle-based ID infusion set (Becton Dickinson Research Catheter; BD Technologies, Durham, NC). The study was designed to determine whether infusion of a placebo solution, insulin diluent, into the dermis using the ID infusion set could be maintained for 24 hours under ambulatory conditions when used in conjunction with commercial insulin pumps. To evaluate and compare the flow performance of the ID infusion set, and because of the insensitivity of current pump alarms, a novel flow algorithm was developed based on fluid infusion pressure to measure the time and duration of disrupted fluid flow during “silent occlusions.”
Methods
Subjects
Subjects were healthy, nondiabetic, adults (aged 18-65 years) in stable health with no acute or significant illness. Key exclusion criteria are shown in Table 1. Subjects were replaced if they had an incomplete data set as a result of failed infusion due to set leakage, pump or data logger malfunction, or voluntary withdrawal from the study. The study complied with the Declaration of Helsinki, and conformed to local regulations; all subjects provided informed consent.
Table 1.
Exclusion Criteria.
| Criteria |
|---|
| Factors promoting bleeding during cannula insertion or usea |
| Dermatologic conditions |
| Excessive abdominal hair or skin imperfections in close proximity to injection site |
| Pregnant |
| Self-reported blood borne infections |
Includes antiplatelet or anticoagulant therapy, low-dose aspirin, or a history of bleeding disorders.
Study Design
This was a single-center, 2-arm (ID and SC), randomized, open-label feasibility study conducted in an inpatient setting. Subjects completed a screening visit, a 24-hour in-clinic interventional visit, and a follow-up examination. Each subject participated in 1 of the 2 arms of the study and received 4 simultaneous randomized infusions (either ID or SC) of insulin diluent in the abdomen using 4 independent pump and set combinations during the interventional visit (Figure 1A).
Figure 1.
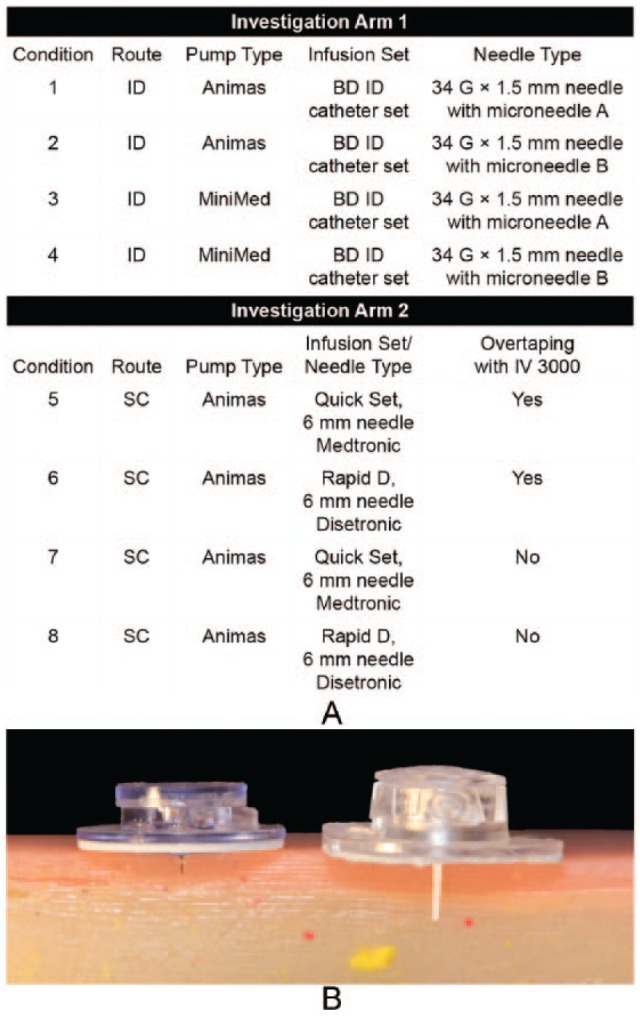
(A) Study design and infusion device combinations used for each investigational arm; (B) 1.5-mm ID steel cannula (left) and 6-mm Teflon SC catheter (right) against a simulated skin model. ID, intradermal; SC, subcutaneous.
In investigational arm 1, ID infusions were administered using microneedle sets, containing a single 34-gauge, 1.5-mm stainless steel infusion cannula and a standard Luer inlet connection (Figure 1B). Two proprietary microneedle cannula designs (versions A and B), having different cannula outlet designs, were each tested using 2 commercial insulin pumps (One Touch® PING® set at the low sensitivity alarm setting, Animas Corporation, West Chester, PA, and its corresponding reservoir or the MiniMed Paradigm 723, Medtronic, Northridge, CA, with a commercially available Luer connect reservoir). Microneedle version A had a more typical needle bevel outlet, while version B incorporated proprietary design features to increase fluid flow. To evaluate ID infusion feasibility across intrinsic pump flow variables, the 2 insulin pumps tested for ID delivery had differing flow profiles during bolus infusion and different pump alarm sensitivities. Tubing lengths for ID and SC sets were a 31 and 43 inches, respectively.
All ID infusion sets were placed using a modified MiniMed Sof-Serter® insertion device (Medtronic, Northridge, CA), and secured with an overtape of IV3000 catheter dressing (Smith and Nephew, London, UK). Investigational arm 2 utilized only the One Touch PING insulin pump, with 2 types of manually inserted SC infusion sets each with a nominal 6-mm insertion depth (Figure 1B): a polymer cannula (Quick-set®, Medtronic, Langhorne, PA), and stainless steel cannula (Accu-chek® Rapid-D, Roche Insulin Delivery Systems, Inc, Fishers, IN). Each SC set type was applied both with and without IV3000 overtaping, for a total of 4 set/tape combinations simultaneously per subject. Overtaping was necessary to maintain long term securement for all ID sets which had a minimal integral adhesive surface due to their investigational design. Overtaping for SC sets was implemented to evaluate whether increasing securement of the device to the skin surface would impact flow and/or leakage performance by reducing relative motion between the set and skin surface. However, incorporation of this variable prevented evaluation of multiple pump types for SC infusion, due to practical limitations on the number of abdominal sites for simultaneous set placement and protocol safety limitations on the allowable number of infusions.
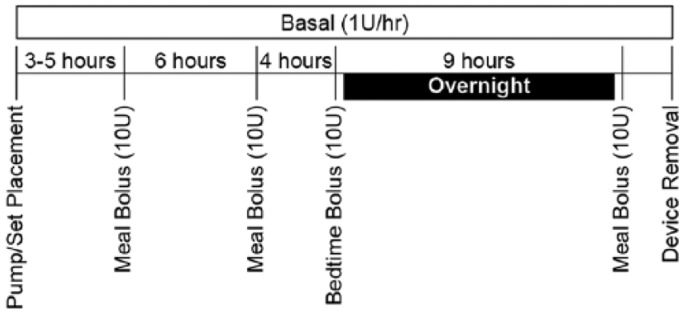
All pumps were programmed to deliver sterile insulin diluent (Eli Lilly, Indianapolis, IN) at a continuous basal delivery rate of 1 unit (10 µL) per hour for 24 hours (Figure 2). Periodic bolus injections of 10 units (100 µL) were administered before each meal and at bedtime, for a total of 4 boli. A fluid pressure transducer (BD-DTX™ Plus, Becton Dickinson, Franklin Lakes, NJ) was connected in-line between the pump reservoir and each infusion set. Two customized dual-channel data logging systems (Device Solutions, Inc, Morrisville, NJ) continuously recorded fluid pressure during infusion for 2 sets each. If an audible pump alarm occurred, that condition was terminated and the device was removed from the subject for the duration of the study. Leakage onto the skin surface was visually observed to the extent possible through the set adhesive during basal and after each bolus delivery. At set removal, any detectable leakage volume was collected from the skin and device surfaces using a cellulose spear and quantified by a validated gravimetric methodology.13
Figure 2.
Study infusion delivery profile.
Endpoints
The primary endpoints were pump occlusion alarms, leakage, and silent occlusion flow interruptions (occurrence rate, time to first occurrence, duration) determined using the proprietary algorithm described below. Secondary endpoints included ID infusion set adhesion performance, tolerability and perceived pain response, infusion site wheal formation and bleeding, and local skin irritation (edema and erythema). Adhesion performance of the ID infusion set was graded as completely adhered, partially adhered, or completely dislodged. Tolerability and pain responses were determined pre- and postbolus using a standard 10-cm numeric visual analog scale (VAS). The tolerability scale ranged from no discomfort (0 cm) to unbearable (10 cm), and the pain scale ranged from no pain (0 cm) to severe pain (10 cm). Wheal formation was documented categorically (ie, yes or no) after removal of the device. Bleeding was evaluated on a scale of 0 to 3 (0 = no bleeding, 1 = just visible spot of red blood, 2 = drop of red blood, 3 = continuous ooze of red blood). Skin irritation was quantified using the Draize dermal irritation scoring system, which ranged from 0 to 4 (0 = no erythema or edema to 4 = severe erythema or edema). Adverse events (AEs) were documented during and after the 24-hour in-clinic interventional visit.
Pressure Algorithm
A proprietary pressure algorithm for flow detection (BD Technologies, Durham, NC) was used to determine whether each micro-pulse during basal infusion resulted in delivery by interrogation of the pressure vs time profiles for each infusion run. Basal micro-pulses occur every 3 minutes at the 1 U/hr rate for both insulin pumps used. Consecutive nondelivery events covering at least 12 minutes (typically 4 micro-pulses) were considered a flow interruption or silent occlusion. The pressure algorithm was developed based on bench-top occlusion data and in vivo pressure data that were correlated to insulin pharmacokinetics during a previous ID basal infusion clinical trial.12 The algorithm14 has good correlation for events that demonstrate increased pressure associated with an occlusion, but has not yet been applied for leakage detection. The output of the algorithm is a list of basal flow interruption events, their duration, and time of occurrence. The algorithm was used to confirm audible pump occlusion alarms in the absence of blood insulin or BG levels, and provide quantitative data on the occurrence of silent occlusion events that occur below the alarm trigger pressures.
Statistical Analysis
The study design included 8 conditions: combinations of 4 infusion sets (ID microneedle A, ID microneedle B, Quickset, Rapid-D), overtaping or not (for SC only), and 2 pump types (for ID only) (see Figure 1). Descriptive statistics were reported for all conditions and endpoints. Statistical comparisons were made to assess the following effects: (1) microneedle design (A vs B, ID route), (2) pump type (Animas vs MiniMed, ID route), (3) commercial infusion set (Quick-set vs Rapid-D, SC route), and (4) overtaping (SC route). In addition, each ID microneedle design was compared to each commercial SC set using infusion data from the Animas pump only. When statistical assumptions allowed, specific comparisons for both main and simple effects were assessed within the context of a single generalized linear model. Alarms were compared using Fisher’s exact test, time to alarm using a Wilcoxon rank sum test, and leakage using a logistic model. Pain, tolerability, bleeding, edema, and erythema were all analyzed with Kruskal–Wallis tests. The number of silent occlusions was compared with a Poisson model, time to first interruption with a linear model, uniformity of interruptions with a test for skewness, and duration/percentage of infusion time interrupted with a repeated measures linear model. Log transformation of the responses was applied as appropriate.
Results
Study Subjects
Forty-three subjects (n = 22 ID infusion; n = 21 SC infusion) were enrolled and randomized, for a total of 172 infusions. For some endpoints, some data were not evaluable because of download errors from the data logger (n = 6 infusions), premature infusion termination due to infusion sets snagging on clothing or other objects (n = 4 infusions), study protocol deviation (n = 4 infusions), or suspected hardware failure (n = 1 infusion). The analysis population consisted of 22 men and 21 women; mean (SD) age of 38.4 (10.3) years. Subjects were 74% white (n = 32), 23% black or African American (n = 10), and 2% Latino (n = 1). Mean (SD) body weight, body mass index, and height were 196.6 (45.3) pounds, 30.6 (7.0) kg/m2, and 67.2 (3.7) inches, respectively.
Silent Occlusions
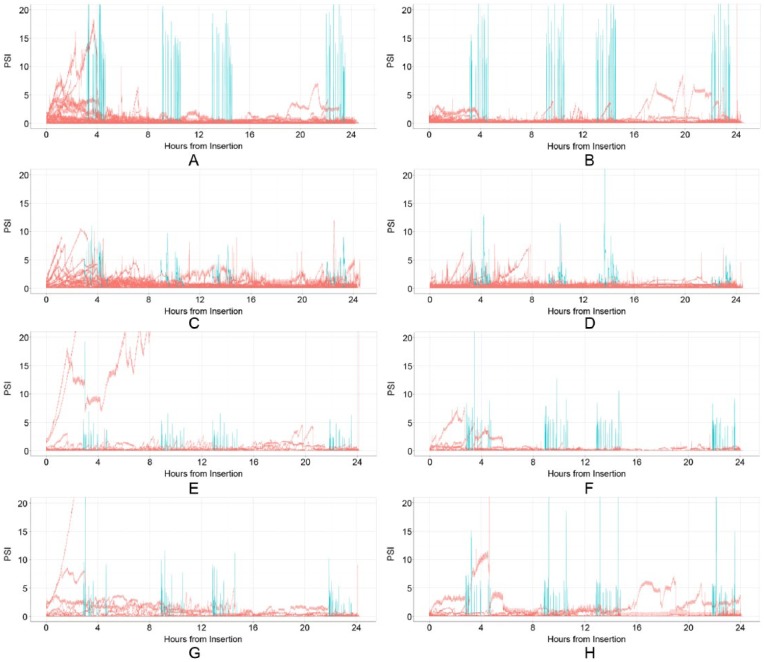
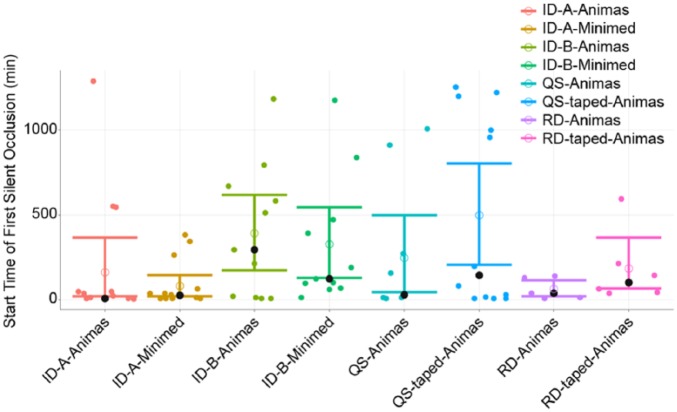
Comprehensive pressure profiles during the 24-hour period of diluent delivery are shown in Figure 3 for each condition. Silent occlusion event endpoints from pressure algorithm interrogation of the pressure–time profiles are presented in Table 2. Microneedle cannula design B had significantly fewer silent occlusion events than microneedle A (P < .01) across insulin pump types. Silent occlusions tended to occur more commonly during initial delivery immediately after device placement than later in the infusion for both ID and SC conditions. The mean time to a first silent occlusion event occurred later for microneedle design B versus microneedle A when used with the MiniMed (P < .04) and Animas pumps (NS; Table 2 and Figure 4). The mean percentage of total infusion time interrupted by silent occlusions was also lower for microneedle B versus microneedle A, regardless of pump used (P = .01, Figure 5). No other factors were statistically different between microneedle designs and pump type alone was not a significant contributing factor for any silent occlusion events.
Figure 3.
Overlaid infusion pressure vs time profiles for all subjects in a given 24-hour infusion condition (n = 19-21/condition): bolus pressures are shown in blue, basal pressures in red (A) ID microneedle A using the Animas pump; (B) ID microneedle B using the Animas pump; (C) ID microneedle A using the MiniMed pump; (D) ID infusion set microneedle B using the MiniMed pump; (E) Quick-set using the Animas pump with overtaping; (F) Rapid-D using the Animas pump with overtaping; (G) Quick-set using the Animas pump; (H) Rapid-D using the Animas pump. ID, intradermal; psi, pressure per square inch.
Table 2.
Silent Occlusion Endpoints.
| Infusion set/pump (N) | Infusions with ≥1 silent occlusion, n | Mean silent occlusions/infusion (min, max) | Mean time to first silent occlusion, minutes (min, max) | Mean duration of silent occlusions, minutes (min, max) | Silent occlusions lasting >1 hour, n | Mean percentage of infusion time silently occluded, % (min, max) |
|---|---|---|---|---|---|---|
| Intradermal infusions | ||||||
| Microneedle A/Animas (22) | 16 | 1.80 (0, 5) | 164.4 (6.5, 1289) | 37.90 (12, 214) | 6 | 5.90 (0, 38) |
| Microneedle B/Animas (22) | 16 | 1.10a (0, 8) | 391.4 (7.5, 1186) | 22.50 (12, 108) | 4 | 2.40 (0, 32)a |
| Microneedle A/MiniMed (22) | 11 | 2.90 (0, 10) | 80.4 (6.7, 380) | 32.00 (12, 148) | 4 | 16.10 (0, 91) |
| Microneedle B/MiniMed (22) | 11 | 1.00a (0, 4) | 321.9 (12.9, 1179)c | 27.50 (12, 88) | 2 | 2.70 (0, 26)a |
| Subcutaneous infusions | ||||||
| Quick-set/Animas (21) | 10 | 1.20 (0, 7) | 246.1 (8.5, 1007) | 36.20 (12, 173) | 2 | 6.40 (0, 88) |
| Quick-set with tape/Animas (21) | 12 | 1.50 (0, 10) | 499.5 (7.4, 1255) | 73.50 (12, 547) | 2 | 10.90 (0, 98) |
| Rapid-D/Animas (21) | 5 | 0.80b (0, 6) | 6.4 (7.6, 140) | 30.20 (12, 114) | 2 | 1.90 (0, 17) |
| Rapid-D with tape/Animas (21) | 6 | 0.50b (0, 3) | 183.6 (37.7, 597) | 35.70 (12, 123) | 1 | 1.00 (0, 9) |
P ≤ .01 for ID infusion set microneedle B vs A; RCS microneedle B had significantly fewer occurrences.
P = .03 for Rapid-D vs Quick-set; Rapid-D had significantly fewer occurrences.
P = .04 for ID infusion set microneedle B vs A; ID infusion set microneedle B had significantly later time to first occurrence.
Figure 4.
Time to first silent occlusion. A, microneedle A; B, microneedle B; ID, intradermal; QS, Quick-set; RD, Rapid-D. Data shown for each infusion in minutes, given a flow-interruption event occurred, with mean and 95% bootstrap CI. The dark circle represents the median, and the open circle represents the mean. Data based on pressure flow algorithm.
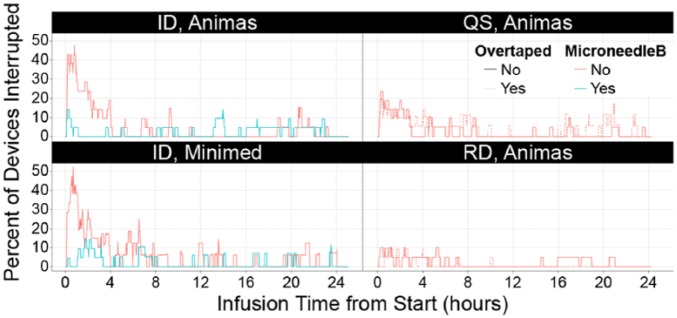
Figure 5.
Percentage of all devices with silent occlusion at a given time. ID, intradermal; QS, Quick-set; RD, Rapid-D. Data are derived from the pressure algorithm.
For SC infusion devices, while silent occlusions were observed for both Rapid-D and Quick-set, Rapid-D had significantly fewer silent occlusions versus Quick-set (P = .03; Table 2). Percentage time interrupted and time to first silent occlusion also showed lower values for Rapid-D, but these and other endpoints compared between SC sets were nonsignificant (P = .08 and 1.0, respectively). Other comparisons including over taping (SC) and pump type (ID) were not significantly different for any silent occlusion measures.
There were no significant differences between either ID microneedle design or the SC Quick-set on any silent occlusion measure. ID microneedle B had similar performance but was nonsignificantly different in comparison to the SC Rapid-D device.
Pump Occlusion Alarms
Among ID devices, an occlusion alarm was significantly more likely to occur with the MiniMed pump (29.5%) than with the Animas pump (0%; P < .001; Figure 6A). The median time to occlusion alarm with the MiniMed pump was 10.3 hours for microneedle B (n = 7) and 3.6 hours with microneedle A (n = 6). Neither overtaping of SC sets nor type of SC device (Quick-set vs Rapid-D) had a significant impact on infusion alarms. However, alarms were observed with both the Quick-set (10%) and Rapid-D (2%) devices during the course of infusion.
Figure 6.
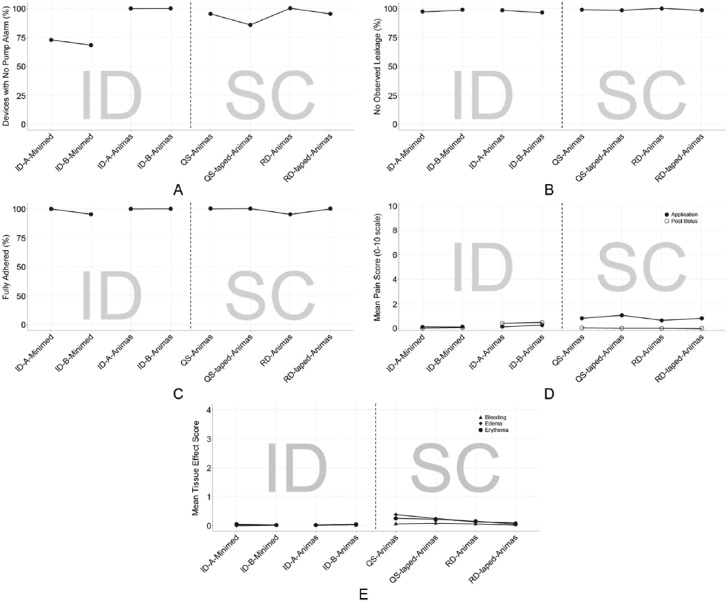
Mean data for study endpoints across various device combinations. Data are presented as the percentage of devices (A) without pump occlusion alarms, (B) without observed leakage, and (C) with full adhesion; (D) mean VAS pain scores at application and postbolus; (E) mean skin reactivity and bleeding scores. For skin reactivity, edema and erythema were measured on an escalating 0-4 scale and bleeding on a 0-3 scale. ID, intradermal; QS, Quick-set; RD, Rapid-D; SC, subcutaneous; VAS, visual analog scale.
Leakage, Adhesion, and Infusion Site Effects
Leakage was observed no more than 4% of the time in any condition, based on observations before and after each bolus and at removal (Figure 6B). Upon removal, 5 ID and 2 SC devices had measureable leakage with maximum amounts of 5.3 uL and 18.4 uL (0.5 and 1.8 U volume equivalent), respectively. Of the 75 ID infusion devices that were not removed due to occlusion alarm, 4 were dislodged due to snagging and removed and 71 remained in place through the 24-hour period. Of those 71, 4 were observed to have partial adherence at some point during the infusion, but were not removed (Figure 6C). Patient-reported mean VAS pain scores did not exceed 1 (0 = no pain) for any condition or time point (Figure 6D). At application, significantly less pain was observed with the ID versus SC devices (Animas pump only, mean 0.2 (ID) vs 0.8 (SC) P ≤ .01). For the first 2 boluses, significantly more pain was observed with ID infusion sets using the Animas pump than with the MiniMed pump (P ≤ .003) or SC infusions (P < .01). Pain scores decreased by the third and fourth boluses, such that differences between ID and SC sets were no longer significant. Tolerability scores were not significantly different between any conditions. ID infusion with the either microneedle cannula design using the Animas pump resulted in lower bleeding scores than either SC infusions; findings were significant for microneedle B vs Rapid-D or Quick-set (P ≤ .02; Figure 6E). There was no bleeding associated with the ID infusion sets using the Animas pump for any infusions, while 27% of infusions with Quick-set and 22% of infusions with Rapid-D had some bleeding. Edema rates were significantly lower during infusion with the ID infusion sets compared to SC sets (P = .04). There was no edema associated with the ID infusion sets using the Animas pump for any infusions, while 27% of infusions with Quick-set and 22% of infusions with Rapid-D had some edema. Rapid-D erythema scores were not quite significantly different between ID and SC sets (P = .06); 2% of ID infusions with the Animas pump were associated with some erythema, compared to Rapid-D, 17% for Quick-set and 7% for Rapid-D.
Four subjects (2 ID, 2 SC) experienced site associated nonserious AEs, which included pruritus, erythema, blisters, and urticaria at 1 or more device locations. All events were mild in severity except 1 case of blistering (SC), which was moderate. In all cases, the adhesive from the commercial device or overtape was the probable cause of AEs.
Discussion
Hollow microneedles have been used extensively for bolus ID administration of vaccines, insulin, and other medications, often with enhanced results on vaccine efficacy and drug pharmacokinetics.2,15 Faster insulin pharmacokinetics, such as those provided by ID delivery, have been identified as important factors to enable optimal performance of closed-loop artificial pancreas systems.16 Although various methods of speeding insulin absorption are under investigation,17 it is unclear whether these methods can reproduce near-physiologic basal and postprandial glycemic control, either alone or in combination with closed loop artificial pancreas systems.18 Previous results from our laboratory have demonstrated basal ID microinfusion for up to 16 hours in a nonambulatory clinical environment.12 The findings reported herein demonstrate the clinical feasibility and reliability of ID microneedle infusion sets to provide both basal and bolus infusion over an extended 24-hour duration using commercial insulin pumps under ambulatory conditions.
Tissue effects (eg, site redness, irritation, bleeding, and possible infection) are common during CSII usage, occurring as rapidly as within 1-2 days of set placement.9 Despite the potential for increased tissue response, ID placebo infusion exhibited equivalent dermal erythema scores, with fewer bleeding incidences than classical SC delivery. Furthermore, there were no discernible increases in dermal edema effects compared to traditional SC infusion. The similarity in observable tissue effects would seem promising for longer term ID infusion usage, but would likely require further clinical confirmation for multiple exposure effects such as scarring or dermal irritation already associated with extended and repetitive CSII usage. There was a slight initial increase in perceived ID bolus pain, but this subsided over time after subsequent deliveries, while ID ranked lower in perceived pain for initial cannula placement. Overall perception of ID infusion was as satisfactory as SC, with both minimal VAS and tolerability scores.
Maintaining successful set adhesion and placement depth within the tissue during use is a known difficulty even using current SC sets19,20 having 4-6 times the intended insertion length (6-9 mm) of the ID sets used herein. Despite this minimal insertion depth, microneedle set adhesion and fluid delivery was readily maintained using adjunctive overtaping as evidenced by the low scores for observed dislodgment and measurable leakage. Overtaping and use of set line safety loops is also recommended for maintaining SC set placement.20 While this procedure had no discernible benefit for SC adhesion or flow performance during the current study, it also had no detectable negative consequences on device performance.
While a variety of factors can contribute to occlusions, such as the insulin analog used, set length, infusion rate, and pump type, there has been limited correlation between any of these factors and the development of an occlusion.5,21 Importantly, intermittent hyperglycemia remains an issue with CSII, in part owing to mechanical problems with the infusion sets,22 underscoring the need for continual technological advances in insulin delivery and failure detection. In this study, there was no statistical difference in the number of occlusion alarms between ID and SC devices. Currently, the MiniMed pump may be less compatible with ID delivery and triggered more pressure alarms, probably due to a lower absolute alarm threshold (max 13.7 psi)23 coupled with higher ID delivery pressures, especially during bolus administration pulses (Figure 3). Mean ID bolus pressures were increased with the Animas pump due to the more rapid and higher volume bolus micro-pulses, but conversely, triggered no pump alarms. This is likely due to the higher Animas alarm threshold setting (approx max 35-40 psi). Overall, the low mean basal infusion pressures encountered during ID delivery were similar to those for SC, and the commercial pumps were readily able to achieve the required basal and bolus delivery pressures. Since ID bolus infusion pressure can vary with injection site location (eg, thigh vs abdomen) and between individuals (unpublished data), these biomechanical factors will need to be considered as system requirements for routine insulin delivery applications. However, in clinical studies to date this has not created any deleterious effect on insulin delivery kinetics, bioavailability, or insulin stability.2-4 Routine ID infusion pump usage could be facilitated by a selectable alarm sensitivity option, much like the high/low sensitivity alarm setting found on some current commercial insulin pumps.
Qualitative issues with insulin pump alarms (eg, false alarms due to threshold settings) have been cited in continuous glucose monitoring insulin studies.24,25 However, quantitative flow performance data observed in this study suggests that alarms were not triggered in a consistent and timely manner in the face of increased in-line pressure. Improved alarms could reduce use of health care resources if under-delivery can be avoided or addressed in a timely fashion. Because of the relative insensitivity of current insulin pump occlusion alarms and the difficulty of performing real time observation of infusion sites in situ, a new method to quantify and compare infusion set flow performance was developed. The in-line pressure monitoring system and algorithm used for this study allows comparative assessment of set function, and has been adapted in our laboratory to provide high sensitivity real-time flow performance monitoring. Use of this method provides quantification for number, duration, and percentage infusion time interrupted by silent occlusions, that occur below typical alarm thresholds. Most investigational CSII fault detection methods are based on interrogating blood glucose profiles, which can be affected by numerous factors beyond set performance.26 To that end, the algorithm presented here provides a method for evaluating pump/set flow performance with respect to these silent occlusions events, and could enable enhanced performance monitoring during insulin infusion, if it were adapted for routine usage within the set flow path.
The root cause of silent occlusions remains unknown, and may be the result of multiple mechanical, chemical or biological factors encountered during insulin infusion. In the present study, silent occlusions were most common immediately after initial cannula placement. These data are consistent with recent reports that cite up to 15% failure after placement of polymer infusion sets.7 This is also readily apparent when plotting the percentage of devices experiencing a flow interruption across the infusion duration period. However, other time periods such as postbolus or after overnight wear did not show a consistent pattern or trend in silent occlusion or occlusion alarm events (Figure 5). Moreover, silent occlusions appear to occur randomly throughout the infusion period for both ID and SC sets, with detection and prevention of these insulin flow faults presenting a major challenge during routine CSII usage as well as for artificial pancreas development.27
The relative importance of microneedle design on ID infusion flow performance was demonstrated by the observed differences between microneedles of equivalent gauge and length dimensions but with varied orifice designs. Overall, microneedle B flow performance was similar to, and for some endpoints such as bleeding and edema better than, the SC infusion set/pump combinations.
Study limitations to consider when evaluating these data include the use of healthy volunteers who were not accustomed to using insulin delivery systems. Placement of all devices was done by study staff; this should be considered when evaluating subjective outcomes (ie, pain and tolerability). Although similar in physical properties, the use of placebo diluents rather than actual insulin may not be fully representative of insulin infusion for occlusion potential or tissue effects nor can it inform on extended ID insulin kinetics and blood glucose control, which are being addressed in additional clinical trials. The 24-hour infusion cycle testing for device feasibility is less than the typical infusion set duration usage and may not capture all events of interest, or later time-driven changes in performance. Last, both the ID infusion system and pressure algorithm used herein are investigational in nature.
Conclusions
ID basal/bolus infusion using the ID infusion set can reliably deliver insulin diluent to healthy human subjects over a 24-hour time period using commercial insulin pumps. ID microneedle design is critical for maximizing infusion performance, and one of the ID designs tested performed similarly to a commercial SC infusion sets. This demonstration of extended duration feasibility of an ID device brings the promise of faster insulin kinetics using ID infusions closer to reality. A novel pressure flow algorithm was able to effectively quantify delivery and evaluate device performance. Silent occlusions were detectable in commercially available infusion devices, and these events may be a contributing factor to problems associated with continual insulin infusion.
Acknowledgments
Writing support was provided by Danielle Gross, PhD, and Susan E. DeRocco, PhD, of Complete Healthcare Communications, Inc (Chadds Ford, PA) under the direction of the authors. Editorial assistance was provided by Maryann T. Travaglini, PharmD, of Complete Healthcare Communications, Inc, and Christopher Rini of Becton Dickinson, who assisted the authors in reviewing and editing the manuscript.
Footnotes
Abbreviations: AE, adverse event; CSII, continuous subcutaneous insulin infusion; ID, intradermal; SC, subcutaneous; VAS, visual analog scale.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors were employees of Becton Dickinson and Company, Franklin Lakes, NJ when this work was conducted. EM and SK are now employed with Parker Hannifin and MaxPoint Interactive, respectively.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Becton Dickinson and Company, Franklin Lakes, NJ.
References
- 1. Gupta J, Felner EI, Prausnitz MR. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol Ther. 2011;13(4):451-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McVey E, Hirsch L, Sutter DE, et al. Pharmacokinetics and postprandial glycemic excursions following insulin lispro delivered by intradermal microneedle or subcutaneous infusion. J Diabetes Sci Technol. 2012;6(4):743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pettis RJ, Ginsberg B, Hirsch L, et al. Intradermal microneedle delivery of insulin lispro achieves faster insulin absorption and insulin action than subcutaneous injection. Diabetes Technol Ther. 2011;13(4):435-442. [DOI] [PubMed] [Google Scholar]
- 4. Pettis RJ, Hirsch L, Kapitza C, et al. Microneedle-based intradermal versus subcutaneous administration of regular human insulin or insulin lispro: pharmacokinetics and postprandial glycemic excursions in patients with type 1 diabetes. Diabetes Technol Ther. 2011;13(4):443-450. [DOI] [PubMed] [Google Scholar]
- 5. van Bon AC, Dragt D, DeVries JH. Significant time until catheter occlusion alerts in currently marketed insulin pumps at two basal rates. Diabetes Technol Ther. 2012;14(5):447-448. [DOI] [PubMed] [Google Scholar]
- 6. van Bon AC, Bode BW, Sert-Langeron C, DeVries JH, Charpentier G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2011;13(6):607-614. [DOI] [PubMed] [Google Scholar]
- 7. Patel PJ, Benasi K, Ferrari G, et al. Randomized trial of infusion set function: steel versus Teflon. Diabetes Technol Ther. 2014;16(1):15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunton S. Insulin delivery systems: reducing barriers to insulin therapy and advancing diabetes mellitus treatment. Am J Med. 2008;121(6 suppl):S35-S41. [DOI] [PubMed] [Google Scholar]
- 9. Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2012;6(4):954-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Jetley R, Jones PL, Ray A. Generic safety requirements for developing safe insulin pump software. J Diabetes Sci Technol. 2011;5(6):1403-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Jones PL, Klonoff DC. Second insulin pump safety meeting: summary report. J Diabetes Sci Technol. 2010;4(2):488-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keith S, McVey E, Pettis RJ. High sensitivity occlusion detection using fluid pressure monitoring during basal insulin infusion. Diabetes. 2013;62 (suppl 1):A249-A250. [Google Scholar]
- 13. Laurent PE, Pettis R, Easterbrook W, Berube J. Evaluating new hypodermic and intradermal injection devices. Med Device Technol. 2006;17(2):16-19. [PubMed] [Google Scholar]
- 14. US Patent Application. 20140107613. System and method for detecting occlusions in a medication infusion system using pulsewise pressure signals. [Google Scholar]
- 15. Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7(7):385-395. [DOI] [PubMed] [Google Scholar]
- 17. Peyser T, Dassau E, Breton M, Skyler JS. The artificial pancreas: current status and future prospects in the management of diabetes. Ann NY Acad Sci. 2014;1311:102-123. [DOI] [PubMed] [Google Scholar]
- 18. McCall AL, Farhy LS. Treating type 1 diabetes: from strategies for insulin delivery to dual hormonal control. Minerva Endocrinol. 2013;38(2):145-163. [PMC free article] [PubMed] [Google Scholar]
- 19. Shetty G, Wolpert H. Insulin pump use in adults with type 1 diabetes—practical issues. Diabetes Technol Ther. 2010;12(suppl 1):S11-S16. [DOI] [PubMed] [Google Scholar]
- 20. American Association of Diabetes Educators. Insulin pump therapy: Best practices in choosing and using infusion devices. 2011. [Google Scholar]
- 21. Kerr D, Morton J, Whately-Smith C, Everett J, Begley JP. Laboratory-based non-clinical comparison of occlusion rates using three rapid-acting insulin analogs in continuous subcutaneous insulin infusion catheters using low flow rates. J Diabetes Sci Technol. 2008;2(3):450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ponder SW, Skyler JS, Kruger DF, Matheson D, Brown BW. Unexplained hyperglycemia in continuous subcutaneous insulin infusion: evaluation and treatment. Diabetes Educ. 2008;34(2):327-333. [DOI] [PubMed] [Google Scholar]
- 23. Medtronic. Paradigm® REAL-Time Revel Insulin Pump MiniMed user guide. 2009. [Google Scholar]
- 24. Wadwa RP, Fiallo-Scharer R, Vanderwel B, Messer LH, Cobry E, Chase HP. Continuous glucose monitoring in youth with type 1 diabetes. Diabetes Technol Ther. 2009;11(suppl 1):S83-S91. [DOI] [PubMed] [Google Scholar]
- 25. Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125-1130. [DOI] [PubMed] [Google Scholar]
- 26. Cameron F, Buckingham BA, Wilson DM, Bequette BW. Extending threshold-based detection of infusion set failures. J Diabetes Sci Technol. 2012;7(1):A17. [Google Scholar]
- 27. Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control. 2012;36(2):255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]