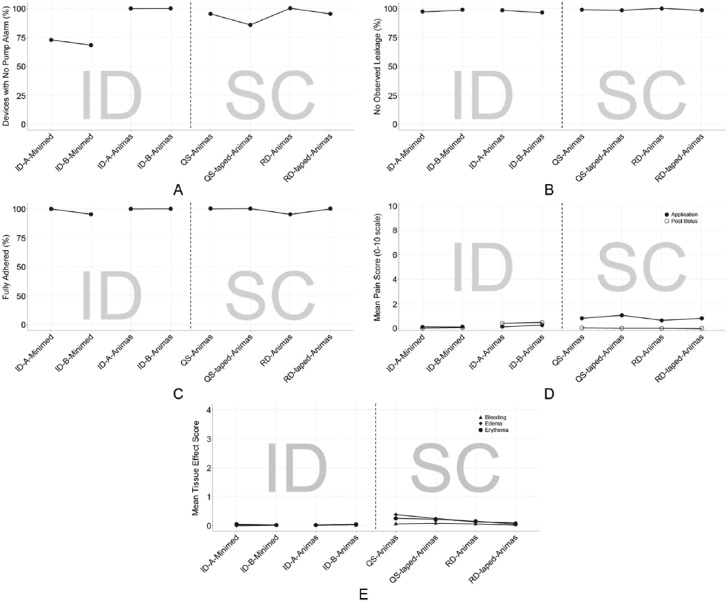
Figure 6.
Mean data for study endpoints across various device combinations. Data are presented as the percentage of devices (A) without pump occlusion alarms, (B) without observed leakage, and (C) with full adhesion; (D) mean VAS pain scores at application and postbolus; (E) mean skin reactivity and bleeding scores. For skin reactivity, edema and erythema were measured on an escalating 0-4 scale and bleeding on a 0-3 scale. ID, intradermal; QS, Quick-set; RD, Rapid-D; SC, subcutaneous; VAS, visual analog scale.